Because steroids and nonsteroidal anti-inflammatory drugs are not ideal for the treatment of corneal neovascularization, researchers are studying the efficacy of bevacizumab (Avastin) and ranibizumab (Lucentis) for treating this disease process.
“We don’t currently have a great answer for corneal neovascularization,” says Terry Kim, MD, professor of ophthalmology at the Duke University Eye Center. “There are downsides to the long-term use of the current medications. For example, steroids can cause high intraocular pressure and cataract formation, and NSAIDs can potentially be toxic to the ocular surface. At least for corneal applications, the attraction of using an anti-VEGF agent, either topically or by subconjunctival injection, is perhaps to better address neovascularization without the long-term side effects of other medications, and perhaps it will be more effective.”
To date, most of the studies on bevacizumab that can be found in the literature are anecdotal reports or were conducted on animals, so some unanswered questions about its use remain. “We still need to figure out the best way to deliver the drug,” Dr. Kim adds. “Is it topically? Is it subconjunctivally? Is it through a scleral contact lens? Is it by injection into the corneal stroma? What is the best formulation or dosing for this application? What is the best drug? There are a host of anti-VEGF agents. We are most familiar with Avastin, but Lucentis has a smaller molecular size and may penetrate the cornea better. How long do we need to apply the drug, and how safe is it?”
Malik Kahook, MD, agrees, noting that it has not yet been determined which disease process would benefit most from this type of therapy. “Because there are so few studies at this point, the surgeons who are treating corneal disease don’t really know how to incorporate Avastin into their regular treatment algorithm,” says Dr. Kahook, an associate professor at the University of Colorado Hospital Eye Center. “We need a larger, randomized study that would convince physicians that there truly is an effect from topical administration or subconjunctival injection of Avastin.”
However, if proven to be safe and efficacious, bevacizumab has the potential to treat patients with a variety of conditions. One of the primary reasons for corneal transplant failure, particularly in the pediatric population, is corneal neovascularization that eventually leads to graft rejection. “Advantages would be a decrease in the neovascularization that happens post-corneal transplant, which could then potentially increase the survival rate or decrease the rejection rate of transplantation in specific patient populations. It would also apply to post-herpetic keratopathy patients, who tend to have higher rates of rejection,” Dr. Kahook says.
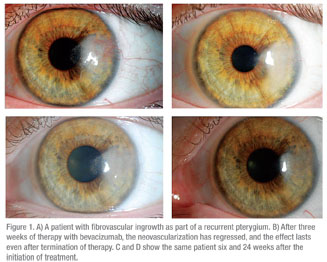 “If it works and if it’s safe, it could really change the way we treat a lot of corneal diseases,” says Richard Davidson, MD, an associate professor of cornea, external disease and refractive surgery at the Rocky Mountain Lions Eye Institute. “In corneal transplant patients, the less neovascularization that is present, the clearer the cornea, so the better the vision is going to be and the lower the risk of rejection.”
“If it works and if it’s safe, it could really change the way we treat a lot of corneal diseases,” says Richard Davidson, MD, an associate professor of cornea, external disease and refractive surgery at the Rocky Mountain Lions Eye Institute. “In corneal transplant patients, the less neovascularization that is present, the clearer the cornea, so the better the vision is going to be and the lower the risk of rejection.”
Bevacizumab could also potentially be used in patients who have neovascularization of the cornea with herpetic keratitis. “Herpes can often result in scar formation and corneal neovascularization,” says Dr. Davidson. “If these patients could be started on Avastin early, then it might even be preventative in that case. Or, if the vessels regressed, the patient may possibly experience clearer vision, and it may reduce some of the scar formation that occurs and possibly even reduce the risk of recurrence.”
Neovascularization is also often seen in people who have sustained chemical injuries. “However, that’s a bit of a double-edged sword,” Dr. Davidson says. “I don’t know that Avastin would be as effective in that situation because the other issue we’re dealing with in these cases is limbal stem cell failure. I think Avastin needs to be used in situations where there is just primary neovascularization without stem cell failure.”
Anti-VEGF agents could potentially be used to treat any condition in which a patient has significant inflammation that results in neovascularization of the cornea. According to Dr. Davidson, this can occur with Salzmann’s nodular degeneration, and it can also be seen following extensive contact lens wear.
Dosage and Delivery
Recent studies have evaluated various dosages and delivery methods of bevacizumab. In an animal model, 5 mg/mL of bevacizumab delivered subconjunctivally was found to be effective in reducing corneal neovascularization and in reducing VEGF levels. The study included the right eyes of 24 rabbits that had sustained an alkaline burn. The eyes were divided into four groups: Group 1 received topical bevacizumab three times daily; group 2 received 5 mg of bevacizumab subconjunctivally every two days; group 3 received 10 mg of bevacizumab subconjunctivally every two days; and group 4 received 0.2 cc of saline. All eyes were treated for seven days.
In group 1, the neovascular index was lower than that in the control group after seven days of topical bevacizumab treatment. In groups 2 and 3, the neovascular index was lower than that in the control group two days after treatment. In the control group, the VEGF level in aqueous humor increased by 66 percent from day seven to 14, while it decreased 49.8 percent in group 1, 70.2 percent in group 2, and 76.4 percent in group 3. According to the authors of the study, “further research is needed to assess the potential side effects and minimal effective dose.”1
Another study confirmed that this dosage is effective. The French study found that a 5 mg/day bevacizumab subconjunctival injection, in monotherapy or in combination with dexamethasone, caused short-term involution of corneal neovascularization after corneal alkali burn and that combination treatments may have advantages over monotherapy. In this study, corneal chemical cauterization was performed on 24 rats. They were divided randomly into six groups and received daily subconjunctival injections for seven days of balanced salt solution 0.1 mL, dexamethasone phosphate 4 mg/day, bevacizumab 2.5 mg/day, 3.75 mg/day, 5 mg/day, or bevacizumab 5 mg/day plus dexamethasone phosphate 4 mg/day. The dexamethasone, bevacizumab 5 mg/day, and dexamethasone plus bevacizumab groups demonstrated a significant lowering in corneal opacity score, and the area of corneal neovascularization was significantly reduced compared with the control group.2
Another unknown is the best location for an injection. A recent study conducted on mice sought to assess the efficacy of bevacizumab for reducing corneal neovascularization and to compare the effects of intraocular (anterior chamber or intravitreal) and subconjunctival injections. The study found that injections partially inhibited the growth of corneal neovascularization induced by acute chemical injury. The anterior chamber and intravitreal injections were found to be most effective, while the subconjunctival injections resulted in the earliest peak response.3
Additionally, a study conducted on human eyes found that the effects of subconjunctival injection of bevacizumab in treating corneal neovascularization with lipid deposition were significant in some patients. The study included 18 eyes in 18 patients with lipid keratopathy. Patients received monthly subconjunctival injections of bevacizumab for three to 10 months according to clinical response. After treatment, the change in best-corrected visual acuity was less than two lines, and the extent, centricity, and percentage of involved corneal surface of the corneal lipid deposition decreased significantly. No eyes experienced any side effects.4
As expected, early treatment appears to yield better results than late treatment. In a rabbit model, limbal insufficiency was surgically created, and eyes received subconjunctival injections of 2.5 mg of bevacizumab twice weekly for one month, immediately (early group), one week (mid group), and one month after injury (late group). Investigators evaluated corneal epithelial alterations, stromal opacity, centricity, extent, and percentage of involved corneal surface of the corneal neovascularization. The study found that early and mid bevacizumab injection inhibited corneal neovascularization, epithelial alteration and stromal opacity, but late treatment did not.5
Concerns
In addition to the concerns mentioned above, another major limiting factor is the cost. “A 5-cc vial of Avastin is about $500, and it has to be formulated at a compounding pharmacy, which can be time-consuming and expensive,” Dr. Kim says. “However, I think it remains a viable option, and as we figure out some of the other issues associated with its use, it may be used more frequently. At least it gives clinicians another option.”
Dr. Davidson notes that there is considerable variability as to how it is prepared at different pharmacies. “You may get a lot more or a lot less of the drug as a result, but no one has looked at stability in the bottle. You have to be very careful about how it’s prepared, and we don’t know if it will lose efficacy after sitting around for 30 days,” he says.
Even less is known about the use of ranibizumab (Lucentis) for treating corneal neovascularization. It is less commonly used because it is even more expensive than bevacizumab. “The primary reason we are not seeing the use of topical Lucentis is that a syringe of Lucentis will only last a few days, and it’s just too expensive,” Dr. Kahook says. “Additionally, these agents need to be more user-friendly. A syringe of Avastin is great for an injection but it’s not really good for putting topical drops on the eye. Then, you have to find a way to put Avastin in a drop bottle, which isn’t easy. So basically, you are taking a repackaged formulation of Avastin, because it was taken out of a vial and put into a syringe, and now you are repackaging it again by taking it from a syringe and putting it into a drop bottle.”
Dr. Kim and colleagues were the first to publish a study on the use of topical bevacizumab in human patients with corneal neovascularization.6 In their report, they treated patients with a 1% concentration of bevacizumab for 25 days, and reported successful results in reducing corneal neovascularization with no adverse effects noted. In addition, Reza Dana, MD, has found that topical ranibizumab, while being more expensive, has a quicker onset of action than topical bevacizumab for the suppression of corneal angiogenesis. His study of ranibizumab is ongoing, and results are not yet published. “The main biologic concern is that there have been some data suggesting that VEGF, which these agents are blocking, can play a role in the health of corneal nerves,” says Dr. Dana, director of cornea and refractive surgery at the Massachusetts Eye and Ear Infirmary and professor of ophthalmology at Harvard Medical School. “Therefore, we do not recommend the use of topical anti-VEGF agents in patients with non-healing corneal defects, and we recommend that the agents not be used for longer than three to four weeks maximum.”
Another concern is the potential for adverse events. Little is known about the topical administration of bevacizumab. For example, how long does it remain in the tissue? Is it absorbed by the tissue, or is it a surface effect? “My guess is that it would actually be efficacious for both neovascularization deep in the cornea and on the surface of the cornea because the Avastin molecule can get in past the cornea and into the eye with topical application,” says Dr. Kahook. “We know that injecting Avastin underneath the conjunctiva can actually treat neovascular disease inside the eye. It definitely penetrates, but we just don’t have specific information to show that. We don’t know how many drops are needed per day. We don’t have many dose/titration-type studies, and we don’t know much about the pharmacokinetics or pharmacodynamics when it is topically instilled. There are a lot of obstacles to topical instillation.”
1. Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol 2009;37:730-736.
2. Hoffart L, Matonti F, Conrath J, et al. Inhibition of corneal neovascularization after alkali burn: Comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Experiment Ophthalmol 2010;38:346-352.
3. Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res 2010;35(2):108-115.
4. Chu HS, Hu FR, Yang, CM, et al. Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea 2011;30:60-66.
5. Lin CT, Hu FR, Kuo KT, et al. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci 2010;51(12):6277-6285.
6. DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol 2007;125:834-836.