The use of stem cells in the treatment of retinal degenerative disease has received increasing attention recently.1 This article will describe some of the key concepts behind stem cell therapy and the issues related to potential stem cell treatment in retinal degenerative diseases.
Definitions and Classes
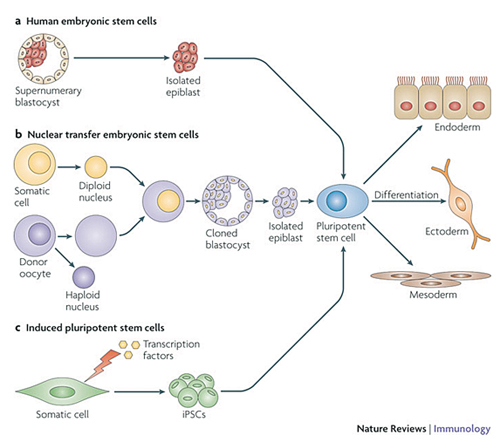
Stem cells are unspecialized cells with the capacity for unlimited self-renewal. Each daughter cell has the capacity to remain a stem cell or to differentiate into more specialized, tissue- or organ-specific cells. Human embryonic stem cells, adult stem cells and induced pluripotent stem cells are considered in detail here (See Figure 1).2
• Human embryonic stem cells (hESCs). hESCs are derived from the inner cell mass of the blastocyst. (The inner cell mass of the three-to-five-day-old, pre-implantation-stage embryo [blastocyst] gives rise to the entire body of the organism.) hESCs are pluripotent, which means they can form all lineages of the body (i.e., ectoderm, mesoderm and endoderm). hESCs can be obtained without destruction of the embryo.3
• Adult (somatic) stem cells. Adult stem cells typically generate the cell types of the tissue in which they reside (See Table 1). Adult stem cells are multipotent, which means they can form multiple cell types of one lineage. For example, a retinal progenitor cell can give rise to photoreceptors, bipolar cells and ganglion cells but not to corneal cells. Adult stem cells are present in many organs and tissues, e.g., brain, bone marrow, teeth, heart, gut, liver, ovarian epithelium and testis. Adult stem cells reside in a specific area of each tissue, termed a “stem cell niche.”4,5 Adult stem cells may remain quiescent for long periods until activated by a normal need for more cells to maintain tissues, or by disease or injury.
|
Human iPSCs express stem cell markers and can produce cells from all three germ layers. Although iPSCs are pluripotent stem cells, iPSCs and ESCs do differ in some important ways (See Table 2). For example, although human iPSCs and ESCs use the same transcriptional network to generate neurons in response to a given set of morphogens, iPSCs do so with significantly reduced efficiency and increased variability.15 As noted previously,1 iPSCs have the theoretical advantage of not being rejected by the patient from whom they are derived (vs. ESCs, unless the ESCs were harvested from the patient as an embryo), but abnormal gene expression in some cells differentiated from iPSCs (both via a retroviral and episomal approach) can induce a T-cell-dependent immune response in a syngeneic recipient.16 This response is likely due to the abnormal expression of antigens not expressed during normal development or differentiation of ESCs, leading to loss of tolerance.16 Expression of these antigens is a reflection of epigenetic differences (e.g., DNA methylation) between iPSCs and ESCs.14,17-21 Continuous passaging of iPSCs may help attenuate these differences,22 but there are risks associated with this approach, as described below. iPSCs seem to be at greater risk for tumor formation (e.g., due to p53 suppression) than ESCs.
It is important to note that passage of ESCs in culture can lead to alterations in the cells that may render them undesirable for cell therapy. This issue is well illustrated by X chromosome inactivation (XCI). XCI refers to repression of transcription of one of the two X chromosomes in female cells.23 (In contrast to most genes, in which the pattern of DNA methylation is identical on both alleles, genes located on X chromosomes normally have only a single allele methylated.) Female human ESCs exhibit varying degrees of XCI.24 In fact, the state of XCI can vary among subcultures of a single human ESC line.25,26 X-inactive specific transcript (XIST) is a long, noncoding RNA, the expression of which is associated with XCI.27-30 Class I lines have both X chromosomes active (XaXa) and can upregulate XIST during differentiation. Class II lines have one inactive X chromosome (XaXi), and class III lines have one inactive X chromosome and have lost XIST expression, although they maintain XCI. Early-passage human ESCs are more likely to be XaXa.31
In one study involving multiple human iPSC lines, loss of XIST expression was associated with upregulation of X-linked oncogenes, downregulation of tumor suppressor genes, accelerated growth rate in vitro, and poorer differentiation in vivo.32 (In contrast, male human iPSC lines did not overexpress oncogenes and generally resembled class II cells.) Loss of XIST expression in human iPSCs can be associated with prolonged time in culture.33 At this time, it seems reasonable to conclude that class III female human iPSC lines should be avoided for in vivo human therapy.32
|
To improve the safety of iPSCs, modified protocols that do not require c-Myc, Sox-2, and/or Klf4 have been described.41-45 In addition, mouse iPSCs can be created without viral vectors46-49 via repeated transfection of two expression plasmids (i.e., non-integrating vector), one containing the complementary DNAs (cDNAs) of Oct3/4, Sox2, and Klf4 and the other containing the c-Myc cDNA, into mouse embryonic fibroblasts, creating iPSCs without evidence of plasmid integration. Other vector-free methods have been used to reprogram cells to pluripotency, e.g., using modified synthetic mRNA,50 recombinant proteins that can penetrate the plasma membrane of somatic cells,51,52 or exposing somatic cells to ESC-conditioned media,53 which may be safer than using viral vectors to induce reprogramming.50
Human iPSCs might be used to study disease pathogenesis, for high-throughput screening to identify small molecule therapy, as well as for cell-based therapy for regenerative medicine (See Figure 2).54,55 However, with increasing time in culture, epigenetic and transcriptional aberrations have been described in PSCs,33,56 so one must verify that the cultured PSCs exhibit the same phenotype as the somatic tissue from the patient. A number of ocular tissues have been derived from stem cells (See Table 3).
|
Immunological Considerations
The immunology of stem cell transplants has been considered elsewhere1 and has been discussed thoroughly.73 Differentiated progeny of ESCs express MHC class I antigens.74,75 Stem cells generated by somatic cell nuclear transfer are syngeneic to the nuclear donor except for the mitochondrial genes, which are of oocyte origin76 and are a source of minor histocompatibility antigens.77 Disparities at the minor histocompatibility loci alone can provoke rejection of ESC-derived tissue.78 Although iPSCs might be devoid of alloreactivity, if the iPSC harbors a genetic abnormality and if this abnormality is corrected before transplantation into the iPSC donor, then an immune response may occur.73
To circumvent immune rejection of transplanted stem cells, developing banks of donor iPSCs has been proposed,73,79 especially from individuals who are homozygous at some of the major histocompatibility (MHC) loci.80 Because disparities at minor histocompatibility loci can provoke immune rejection, it is not clear that this approach will be useful for many patients. Although MHC matching could be supplemented with immune suppressive therapy, this approach might be accompanied by an increased risk of ESC-derived tumor formation. Other strategies that might be effective include:1
• Treatment with CD4- and CD8-specific antibodies. In preclinical models, tolerance to transplanted ESC-derived tissue can be induced using a short course of non-depleting CD4- and CD8-specific monoclonal antibodies.78,81 Lack of donor dendritic cells in the graft may be critical for the development of tolerance with this approach.
• Co-transplant iPSCs with dendritic cells. iPSCs can provide both immature dendritic cells that express the alloantigens for which tolerance is required as well as the therapeutic tissue of interest.73,82 Administration of immature dendritic cells before transplantation of therapeutic tissue might help induce tolerance to alloantigens.83,84 Exposure of the dendritic cells to pharmacological agents or blockade of amino acid catabolism can enhance their tolerogenicity.79,85-87
• Co-transplant iPSCs with mesenchymal stem cells. Mesenchymal stem cells can induce tolerance to stem cell-derived tissue grafts.88-90
In summary, control of the immune response is likely to be an important aspect of stem cell therapy even if iPSCs are used. As noted elsewhere,1,91 the role of the immune suppressive nature of the subretinal space, as well as the inherent immunological properties of the transplanted tissue (e.g., photoreceptors or RPE cells) in mitigating this requirement is not clear at this time.
Replacement vs. Rescue Therapy
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Replacement
Replacement therapy is an approach to regenerative medicine in which healthy cells replace cells that have died or are dysfunctional. For example, in retinitis pigmentosa, photoreceptors die. Replacement therapy for RP could involve transplantation of cells that can integrate with the host retina and function as photoreceptors. Replacement retinal therapy is sight-restoring.
To be useful for cell replacement therapy, stem cells must proliferate extensively to generate sufficient quantities of material if they are intended to serve as a “universal donor.” If the stem cells are derived from the patient (e.g., iPSCs), then the requirement for extensive proliferation may be reduced considerably since these cells will serve only one recipient. Stem cells must stably differentiate into the desired cell type(s). hESC-derived RPE, for example, can spontaneously dedifferentiate to non-RPE-like cells and spontaneously redifferentiate into RPE-like cells, indicating phenotypic instability.62 The cultures may not retain a stable phenotype after five to eight passages. ESCs and iPSCs vary in their tendency to differentiate into cells of a given lineage.14,39
What defines a “differentiated” RPE cell? Table 4 summarizes a number of potentially important features of differentiated RPE cells.92
What defines a photoreceptor cell? Gene expression profiling has been used to determine how closely ESC-derived retinal cells resemble normal retina, the developmental stage of the ESC-derived cells (relative to fetal retinal cells), and whether there are significant contaminating non-retinal cells.95 These studies indicate that some minimal contamination with non-retinal cells (e.g., RPE, ciliary epithelium) can occur, but that undifferentiated, pluripotent cells decline with time in culture, which may mean that a longer duration differentiation protocol may minimize the risk of teratoma formation. Some features of photoreceptor differentiation rely on interactions with surrounding cells. Interaction of photoreceptors with RPE is critical for foveal development.96 Interaction with Müller cells via crumbs homolog 1 protein (a constituent of the zonula adherens) is important for normal outer retinal organization.97
| ||||||||||||||||
Transplanted cells must integrate into the surrounding tissue for replacement therapy to succeed. Targeted disruption of glial reactivity and disruption of the outer limiting membrane may improve integration of transplanted cells.104-106 The developmental age of the donor cells may be critical for successful integration with host retina,107 but it is not clear that this is the case.108 The synaptic reorganization that accompanies photoreceptor degeneration in RP109 might limit the extent of functional transplanted photoreceptor integration with the host. Presumably, if the transplanted cells have differentiated and integrated appropriately, they also will function physiologically in the host tissue. Relatively few functioning cones are needed to sustain visual acuity of 20/30.110
Rescue
Rescue refers to the preservation and, in some cases, restoration of function of tissue that is destined to die or malfunction due to an underlying disease. Cells that mediate rescue may elaborate needed trophic factors and must not proliferate in an uncontrolled manner. Rescue therapy may be sight-restoring to the degree that dying cells, which cannot support vision, can return to normal physiological function. Degenerating photoreceptors, for example, may first lose their outer segments, and thus become inefficient transducers of light energy. Under these circumstances, if rescue therapy restores elaboration of outer segments, vision might improve.
| ||||||||||||||||||||
Although consideration of iPSCs as screening tools is beyond the scope of this brief review, one might note that iPSCs harbor the disease-associated genes of the patient from which they are derived. Cultures of these cells might be used for high-throughput screening to identify small molecules that reverse or halt the biochemical abnormalities induced by the disease-causing mutation(s). In this sense, the iPSCs can serve as a basis for developing pharmacological treatments for degenerative retinal diseases.
Combined Replacement/Rescue
Diseases in which RPE cells appear to be targeted primarily include Best disease116,117 and some forms of RP,118,119 and secondarily include Stargardt macular dystrophy120,121 and AMD.40,122 RPE cell transplants are an attractive starting point for cell-based combination replacement and rescue therapy in the eye because hESCs and iPSCs can be induced to differentiate into RPE relatively easily, and one can generate large quantities of cells with stable genotype and appropriate phenotype. Currently, RPE differentiation from ESCs is a two-step process, typically requiring weeks in culture.62,123,124 The first step involves converting ESCs or iPSCs into cells with neuroectodermal properties. The second step involves differentiating neuroectodermal cells into RPE cells. At this time, only a part of the ESC or iPSC culture is transformed into RPE, which may mean that the cultures are heterogeneous. hESC-derived RPE tends to resemble fetal RPE more closely than adult RPE, and iPSC-derived RPE seems to be in a unique differentiation state.62,68,125-127 Both hESC- and iPSC-derived RPE express differentiation markers: tryosinase (melanin); premelanosomal protein-17 (melanin); Bestrophin-1 (chloride channel); MERTK (phagocytosis); focal adhesion kinase; PEDF/VEGF (growth factors); and RPE65/RLBP1 (visual cycle).
In addition to the relative ease of producing differentiated RPE from stem cell progenitors, RPE cells integrate easily with host photoreceptors, and RPE cells elaborate trophic substances that support photoreceptors.100,128,129 There is robust evidence for RPE transplant efficacy in pre-clinical models.91 However, in the case of AMD eyes, survival and proper differentiation on submacular Bruch’s membrane may be problematic.100
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Retinal Degenerative Disease
Stem cell therapy has been effective in preclinical models of retinal degenerative disease, including models of RP and Stargardt macular dystrophy (See Table 5).
Stem cells are being used in human clinical trials to treat degenerative retinal diseases, including Stargardt macular dystrophy, AMD and RP (See Table 6). These studies represent early efforts in this area. Two of the studies have published preliminary results, and these studies are considered in greater detail below.
|
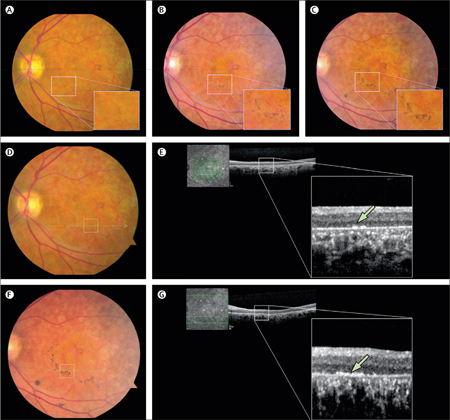
Stargardt macular dystrophy is the most common macular dystrophy of childhood.140 Currently, gene therapy (clinicaltrials.gov identifier: NCT01367444; sponsor: Oxford BioMedica) and nutritional supplementation (NCT01278277; sponsor: Catholic University of the Sacred Heart, Rome) also are under study for this condition. Experiments in an animal model of Stargardt disease indicate that hESC-derived RPE can rescue photoreceptors.125 In one study, four months after subretinal transplantation of hESC-derived RPE into a patient with advanced Stargardt macular dystrophy, visual acuity improved from hand motions before surgery to 20/800.141 There was no improvement in the unoperated fellow eye. Pigmented cells at the transplant site seemed to proliferate during the four-month period of observation. Optical coherence tomography indicated that the pigmented cells were organized in a monolayer. OCT images of the retina overlying these cells did not demonstrate improved photoreceptor anatomy, and the subjacent choroid seemed unchanged also (See Figure 3). There was no evidence of teratoma formation or immune rejection of the transplanted cells. This patient is enrolled in a Phase I/II open-label, prospective, multicenter study to determine the safety and tolerability of subretinal transplantation of hESC-derived RPE cells in patients with Stargardt macular dystrophy and AMD. As part of the treatment protocol, the patient received a seven-week course of tacrolimus and mycophenolate mofetil starting one week before surgery. Per protocol, at week six after surgery, tacrolimus was discontinued and mycophenolate mofetil was continued for an additional six weeks. It is not clear whether the immune suppressive subretinal space91 will prevent rejection of these HLA Class II expressing cells after immune suppression is stopped.
AMD
AMD is the leading cause of blindness in persons older than 55 years in the United States.142 AMD can cause visual loss through two mechanisms: choroidal new vessels and geographic atrophy. Although there are effective treatments for CNVs,143 there is no proven therapy for GA currently. A number of potential treatments are under study, and they are listed in Table 7.143
One group has reported that four months after subretinal transplantation of hESC-derived RPE into a patient with GA, vision improved from 20/500 at entry to 20/200 by week two after surgery.141 Visual acuity was 20/320 by week six and remained stable at the three-month follow-up visit. Of note, mild visual improvement was also noted in the unoperated fellow eye after surgery. This patient received tacrolimus and mycophenolate mofetil as described above.
Retinitis Pigmentosa
In a prospective Phase I, nonrandomized open-label study of RP patients with best-corrected ETDRS visual acuity worse than 20/200,139 three patients with RP and two with cone-rod dystrophy underwent intravitreal injection of autologous bone marrow-derived mononuclear cells with no adverse effects (and no documented benefit at 10-months follow-up).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Issues Remain
In contrast to standard pharmacological monotherapy, transplanted cells can secrete numerous molecules that may exert a beneficial effect on the host retina and/or choroid even if they do not cure the underlying disease101,125,128,129,144 As noted previously, with a single transplant operation, many different pathways can be modified, which may reduce the chance of “escape” associated with monotherapy as well as the need for repeated drug administration. In addition, transplanted cells can replace dead cells (e.g., photoreceptors). Due to their pluripotency and unlimited proliferative capacity, stem cells seem to be the best starting material for cell-based therapy because these cells can be produced en masse safely, and they can be induced to differentiate into ocular cells with potential for replacement and rescue therapy. Preclinical studies demonstrate the feasibility of using ESCs and iPSCs for treating degenerative retinal diseases associated with abnormalities in the RPE and/or photoreceptors.
Some issues, however, may limit the use of stem cells in clinical practice, including: immunogenicity of the cells; stability of cell phenotype (both inherent and environment-induced); propensity of the cells to form tumors in situ; influence of the abnormal microenvironment that can accompany degenerative disease; and synaptic rewiring that accompanies retinal degeneration. In the case of non-exudative AMD, cell transplants might prevent GA progression (through replacement of dysfunctional or dead RPE) and might even bring about some visual improvement in selected cases (through rescue of dying photoreceptors). Cell-based therapy may one day be sight-restoring for patients who are blind due to retinal degenerations of various etiologies. RPE transplantation is an attractive starting point for this sort of therapy, since these cells can integrate with the host retina easily.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Supported in part by Research to Prevent Blindness Inc. and the Joseph DiSepio AMD Research Fund. Contact Dr. Zarbin at the Institute of Ophthalmology and Visual Science, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Room 6155, Doctors Office Center, 90 Bergen Street, Newark, N.J. 07103. Phone: 973-972-2038; fax: 973-972-2068; email:
zarbin@umdnj.edu.
1. Zarbin MA. The Promise of Stem Cells for Age-Related Macular Degeneration and Other Retinal Degenerative Diseases. Drug Discov Today 2012:in press.
2. Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008;132:567-82.
3. Chung Y, Klimanskaya I, Becker S, et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2008;2:113-7.
4. Chen S, Wang S, Xie T. Restricting self-renewal signals within the stem cell niche: Multiple levels of control. Curr Opin Genet Dev 2011;21:684-9.
5. Li L, Xie T. Stem cell niche: Structure and function. Annu Rev Cell Dev Biol 2005;21:605-31.
6. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 1986;103:49-62.
7. Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55.
8. Takeda M, Takamiya A, Jiao JW, et al. alpha-Aminoadipate induces progenitor cell properties of Muller glia in adult mice. Invest Ophthalmol Vis Sci 2008;49:1142-50.
9. Del Debbio CB, Balasubramanian S, Parameswaran S, Chaudhuri A, Qiu F, Ahmad I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. PLoS ONE 2010;5:e12425.
10. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997;385:810-3.
11. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76.
12. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72.
13. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917-20.
14. Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467:285-90.
15. Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 2010;107:4335-40.
16. Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212-5.
17. Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009;5:111-23.
18. Stadtfeld M, Apostolou E, Akutsu H, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 2010;465:175-81.
19. Doi A, Park IH, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 2009;41:1350-3.
20. Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011;471:68-73.
21. Zhao T, Xu Y. p53 and stem cells: new developments and new concerns. Trends Cell Biol 2010;20:170-5.
22. Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 2010;28:848-55.
23. Kim DH, Jeon Y, Anguera MC, Lee JT. X-chromosome epigenetic reprogramming in pluripotent stem cells via noncoding genes. Semin Cell Dev Biol 2011;22:336-42.
24. Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci U S A 2008;105:4820-5.
25. Hall LL, Byron M, Butler J, et al. X-inactivation reveals epigenetic anomalies in most hESC but identifies sublines that initiate as expected. J Cell Physiol 2008;216:445-52.
26. Shen Y, Matsuno Y, Fouse SD, et al. X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc Natl Acad Sci U S A 2008;105:4709-14.
27. Kay GF, Penny GD, Patel D, Ashworth A, Brockdorff N, Rastan S. Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell 1993;72:171-82.
28. Lengner CJ, Gimelbrant AA, Erwin JA, et al. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell 2010;141:872-83.
29. Panning B, Dausman J, Jaenisch R. X chromosome inactivation is mediated by Xist RNA stabilization. Cell 1997;90:907-16.
30. Wutz A, Jaenisch R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell 2000;5:695-705.
31. Dvash T, Lavon N, Fan G. Variations of X chromosome inactivation occur in early passages of female human embryonic stem cells. PLoS ONE 2010;5:e11330.
32. Anguera MC, Sadreyev R, Zhang Z, et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell 2012;11:75-90.
33. Nazor KL, Altun G, Lynch C, et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 2012;10:620-34.
34. Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920-3.
35. Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A 2008;105:5856-61.
36. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448:313-7.
37. Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 2007;1:39-49.
38. Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells 2011;29:825-35.
39. Feng Q, Lu SJ, Klimanskaya I, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 2010;28:704-12.
40. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 2004;122:598-614.
41. Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008;26:101-6.
42. Li W, Zhou H, Abujarour R, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 2009;27:2992-3000.
43. Zhu S, Li W, Zhou H, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010;7:651-5.
44. Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 2008;26:1269-75.
45. Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008;3:568-74.
46. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008;322:949-53.
47. Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797-801.
48. Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009;458:766-70.
49. Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 2009;458:771-5.
50. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010;7:618-30.
51. Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4:381-4.
52. Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009;4:472-6.
53. Balasubramanian S, Babai N, Chaudhuri A, et al. Non cell-autonomous reprogramming of adult ocular progenitors: Generation of pluripotent stem cells without exogenous transcription factors. Stem Cells 2009;27:3053-62.
54. Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 2011;13:497-505.
55. Jin ZB, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS ONE 2011;6:e17084.
56. Mekhoubad S, Bock C, de Boer AS, Kiskinis E, Meissner A, Eggan K. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 2012;10:595-609.
57. Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res 2007;17:26-36.
58. Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res 2009;88:747-51.
59. Chen M, Chen Q, Sun X, et al. Generation of retinal ganglion-like cells from reprogrammed mouse fibroblasts. Invest Ophthalmol Vis Sci 2010;51:5970-8.
60. Parameswaran S, Balasubramanian S, Babai N, et al. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: Therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells 2010;28:695-703.
61. Jagatha B, Divya MS, Sanalkumar R, et al. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochem Biophys Res Commun 2009;380:230-5.
62. Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 2004;6:217-45.
63. Gong J, Sagiv O, Cai H, Tsang SH, Del Priore LV. Effects of extracellular matrix and neighboring cells on induction of human embryonic stem cells into retinal or retinal pigment epithelial progenitors. Exp Eye Res 2008.
64. Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009;5:396-408.
65. Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc 2009;4:811-24.
66. Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett 2009;458:126-31.
67. Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A 2009;106:16698-703.
68. Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 2009;27:2427-34.
69. Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE 2009;4:e8152.
70. Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells 2006;24:246-57.
71. Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 2008;26:215-24.
72. Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 2009;122:3169-79.
73. Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat Rev Immunol 2010;10:868-75.
74. Boyd AS, Wood KJ. Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters. Transplantation 2009;87:1300-4.
75. Tian L, Catt JW, O’Neill C, King NJ. Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells. Biol Reprod 1997;57:561-8.
76. Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science 2001;292:740-3.
77. Fairchild PJ, Robertson NJ, Cartland S, Nolan KF, Waldmann H. Cell replacement therapy and the evasion of destructive immunity. Stem Cell Rev 2005;1:159-67.
78. Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci U S A 2007;104:20920-5.
79. Boyd AS, Fairchild PJ. Approaches for immunological tolerance induction to stem cell-derived cell replacement therapies. Expert Rev Clin Immunol 2010;6:435-48.
80. Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol 2008;26:739-40.
81. Lui KO, Boyd AS, Cobbold SP, Waldmann H, Fairchild PJ. A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across a major histocompatibility complex barrier. Stem Cells 2010;28:1905-14.
82. Choi KD, Vodyanik MA, Slukvin, II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest 2009;119:2818-29.
83. Silk KM, Fairchild PJ. Harnessing dendritic cells for the induction of transplantation tolerance. Curr Opin Organ Transplant 2009;14:344-50.
84. Fairchild PJ, Nolan KF, Cartland S, Waldmann H. Embryonic stem cells: A novel source of dendritic cells for clinical applications. Int Immunopharmacol 2005;5:13-21.
85. Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007;178:7018-31.
86. Horibe EK, Sacks J, Unadkat J, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol 2008;18:307-18.
87. Leishman AJ, Silk KM, Fairchild PJ. Pharmacological manipulation of dendritic cells in the pursuit of transplantation tolerance. Curr Opin Organ Transplant 2011;16:372-8.
88. Ge W, Jiang J, Baroja ML, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 2009;9:1760-72.
89. Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008;181:3933-46.
90. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42-8.
91. Gullapalli VK KM, Wang H, Sugino IK, Madreperla S, Zarbin MA. Retinal Pigment Epithelium and Photoreceptor Transplantation Frontiers. In: SJ R, editor. Retina 4th Edition. Philadelphia: Mosby, Inc., 2006:2597-2613.
92. Bharti K, Miller SS, Arnheiter H. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res 2011;24:21-34.
93. Strunnikova NV, Maminishkis A, Barb JJ, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet 2010;19:2468-86.
94. Wang FE, Zhang C, Maminishkis A, et al. MicroRNA-204/211 alters epithelial physiology. Faseb J 2010;24:1552-71.
95. Lamba DA, Reh TA. Microarray characterization of human embryonic stem cell-derived retinal cultures. Invest Ophthalmol Vis Sci 2011;52:4897-906.
96. Jeffery G. The albino retina: An abnormality that provides insight into normal retinal development. Trends Neurosci 1997;20:165-9.
97. Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res 2008;86:713-26.
98. Kustermann S, Hildebrandt H, Bolz S, Dengler K, Kohler K. Genesis of rods in the zebrafish retina occurs in a microenvironment provided by polysialic acid-expressing Muller glia. J Comp Neurol 2010;518:636-46.
99. Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: Anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol 2008;214:347-61.
100. Sugino IK, Sun Q, Wang J, et al. Comparison of FRPE and human embryonic stem cell-derived RPE behavior on aged human Bruch’s membrane. Invest Ophthalmol Vis Sci 2011;52:4979-97.
101. Chalmel F, Leveillard T, Jaillard C, et al. Rod-derived Cone Viability Factor-2 is a novel bifunctional-thioredoxin-like protein with therapeutic potential. BMC Mol Biol 2007;8:74.
102. Leveillard T, Mohand-Said S, Lorentz O, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet 2004;36:755-9.
103. Yang Y, Mohand-Said S, Danan A, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther 2009;17:787-95.
104. Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci 2010;51:960-70.
105. West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res 2008;86:601-11.
106. Pearson RA, Barber AC, West EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant 2010;19:487-503.
107. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006;444:203-7.
108. Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci 2011.
109. Jones BW, Watt CB, Frederick JM, et al. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol 2003;464:1-16.
110. Geller AM SP. How many cones are required to “see?”: Lessons from Stargardt’s macular dystrophy and from modeling with degenerate photoreceptor arrays. In: al JHe, editor. Retinal Degeneration. New York: Plenum Press, 1993:25-34.
111. Bull ND, Irvine KA, Franklin RJ, Martin KR. Transplanted oligodendrocyte precursor cells reduce neurodegeneration in a model of glaucoma. Invest Ophthalmol Vis Sci 2009;50:4244-53.
112. Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci 2010;51:2051-9.
113. Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol 2007;245:414-22.
114. Inoue Y, Iriyama A, Ueno S, et al. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res 2007;85:234-41.
115. Sugino IK, Sun Q, Wang J, et al. Comparison of fetal RPE and human embryonic stem cell derived-RPE (hES-RPE) behavior on aged human Bruch’s membrane. Invest Ophthalmol Vis Sci 2011.
116. Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A 2000;97:12758-63.
117. Bakall B, Radu RA, Stanton JB, et al. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp Eye Res 2007;85:34-43.
118. Morimura H fG, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. 1998;95:3088-3093.
119. Maw MA, Kennedy B, Knight A, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet 1997;17:198-200.
120. Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997;15:236-46.
121. Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A 2000;97:7154-9.
122. Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog Retin Eye Res 2010;29:95-112.
123. Klimanskaya I. Retinal pigment epithelium. Methods Enzymol 2006;418:169-94.
124. Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: Sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov 2008;7:131-42.
125. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009;27:2126-35.
126. Carr AJ, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis 2009;15:283-95.
127. Liao JL, Yu J, Huang K, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet 2010;19:4229-38.
128. Kolomeyer AM, Sugino IK, Zarbin MA. Characterization of conditioned media collected from cultured adult versus fetal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2011;52:5973-86.
129. Lund RD, Adamson P, Sauve Y, et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc Natl Acad Sci U S A 2001;98:9942-7.
130. Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 2004;114:765-74.
131. Stitt AW, O’Neill CL, O’Doherty MT, Archer DB, Gardiner TA, Medina RJ. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res 2011;30:149-66.
132. Meyer JS, Katz ML, Maruniak JA, Kirk MD. Neural differentiation of mouse embryonic stem cells in vitro and after transplantation into eyes of mutant mice with rapid retinal degeneration. Brain Res 2004;1014:131-44.
133. Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells 2006;24:274-83.
134. Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 2009;4:73-9.
135. Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 2006;8:189-99.
136. Gamm DM, Wang S, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE 2007;2:e338.
137. Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 2007;25:602-11.
138. Lu B, Wang S, Francis PJ, et al. Cell transplantation to arrest early changes in an ush2a animal model. Invest Ophthalmol Vis Sci 2010;51:2269-76.
139. Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A phase I trial. Retina 2011;31:1207-14.
140. Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet 2009;30:63-8.
141. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012.
142. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564-72.
143. Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: An integrated survey of emerging treatment alternatives. Retina 2010;30:1350-67.
144. Kolomeyer AM, Sugino IK, Zarbin MA. Characterization of conditioned media collected from aged versus young human eye cups. Invest Ophthalmol Vis Sci 2011;52:5963-72.
145. Wang NK, Tosi J, Kasanuki JM, Chou CL, Kong J, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation 2010;89:911-9.