The vast majority of both unilateral and bilateral ptosis is due to levator dehiscence or laxity. However, on rare occasions, ptosis may be associated with significant systemic or intracranial disease. In addition to the usual measurements that are documented in all ptosis patients (margin to reflex distance, levator excursion, tear function, etc.), several specific items need to be clearly documented in the history and physical in every patient presenting with ptosis.
As a succinct but admittedly oversimplified statement, there are five potentially dangerous disease entities that may present with unilateral or bilateral ptosis:
- Horner syndrome;
- Partial or complete CN-III palsy;
- Myasthenia gravis;
- Malignancy or infection of the superior eyelid, conjunctival cul-de-sac or anterior orbit; and
- Chronic progressive external ophthalmoplegia.
To avoid missing a potentially devastating disease process manifesting as ptosis, the clinician must follow a relatively straightforward rule in ptosis evaluation: Always completely examine and document the triad of lid position, pupillary size/reactivity and extraocular motility. In other words, if one is abnormal, you must check the other two.
In this issue, Horner syndrome (HS) and CN-III dysfunction will be discussed. The remaining three will appear in the next installment of Plastic Pointers.
Horner Syndrome
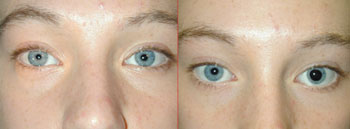
Any patient presenting with unilateral ptosis is suspected of having Horner syndrome (oculosympathetic paresis) until proven otherwise. It is extremely important to document not only the reactivity of the pupils, but also the size of the pupils.
Remember that 20 percent of the general population younger than 18 years has physiologic anisocoria, and this number increases with age: Up to 50 percent of the population older than 65 has subtle physiologic anisocoria. Because of the high incidence of both physiologic anisocoria and ptosis, statistically patients with these two findings will present to the ophthalmologist much more often than patients with true HS.
|
If anisocoria is present, pupillary size should be measured in both light and dark conditions. The relative anisocoria will remain the same if is physiologic, and will increase in darkness in patients with HS (because the miotic side will not dilate as quickly due to sympathetic nerve damage). Two additional clues should be sought. First, check for dilation lag during pupillary exam. In HS, the affected pupil will continue to react briskly to light, but dilate more slowly than the unaffected side. The corollary to this finding is that the measurement of pupils in dim light should be done within seconds of turning the lights down; any significant delay will allow the affected pupil to recover, with a resultant misdiagnosis of “physiologic anisocoria.” In subtle cases, slit-lamp examination of pupillary reactivity may show dilation lag more clearly.
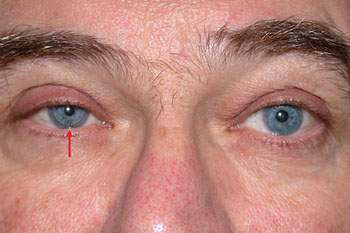
Second, always check the position of the lower lid. In many cases of HS, not only will the upper lid droop, but the lower lid on the affected side will appear higher (“reverse ptosis”) than the unaffected side because of paresis of the inferior tarsal muscle (See Figure 1). A finding of conjunctival hyperemia is helpful when present, but tends to be transient. Anhydrosis may also be present, but is difficult to ascertain by history, and testing is impractical in an ophthalmologist’s office.
If HS is established, the one most important point to document is the presence or absence of pain (headache, brow ache, forehead tingling, neck pain). The full history should elicit:
- pain;
- history of smoking;
- lung or breast cancer;
- recent neck or thyroid surgery;
- recent trauma; and
- duration of ptosis.
A recent study concluded that in most cases of HS, a careful history will lead the clinician to the etiology.
If Horner syndrome is suspected, there are two choices: pharmacologic testing or neuroimaging. The bottom line is that regardless of the outcome of pharmacologic testing, the majority of patients with new-onset HS will require imaging.
Although cocaine and hydroxyamphetamine may be available in many neuro-ophthalmology clinics, these agents are difficult to come by in other offices or emergency rooms, and many would argue that hydroxyamphetamine has become a relatively useless test in the diagnosis and management of HS. Apraclonidine 0.5% is a relatively ubiquitous agent in an ophthalmologist’s office and is easily available.
In cases of HS, apraclonidine instilled in both eyes will result in a slight mydriasis of the affected side and a miosis of the unaffected side (i.e., a reversal of the anisocoria) and in some patients a mild upper lid retraction. However, because apraclonidine relies on the presence of denervation hypersensitivity, which may take weeks to develop, a negative apraclonidine test in acute or subacute situations does not effectively rule out the possibility of HS.
|
Imaging
The type of imaging for HS is directed by the history. If the HS has been present since birth in an older child or adult, no imaging is needed because the lesion is congenital (remember that congenital HS in an infant or acquired HS in a child still requires a radiographic workup). If the patient developed HS immediately following thyroidectomy or other neck/chest procedure, again, no imaging is needed because the location of the injury is obvious. However, the majority of new onset HS needs the following radiologic workup:
- Chest imaging: Plain X-ray or chest CT, with special attention to the lung apices. This is especially critical in patients with a smoking history or those complaining of scapular, shoulder, or arm pain.
- MRI/MRA or CT/CTA of the head.
- MRI/MRA or CT/CTA of the neck. Note that MRI of the head alone is insufficient. Vascular imaging of both the head and neck is necessary in all cases.
Imaging should be performed as quickly as possible (within days). Immediate imaging is required in anyone with new onset (less than four weeks) HS complaining of headache or neck pain to rule out carotid artery dissection (See Figure 2). A delay in diagnosis and treatment may result in hemispheric stroke. If a carotid artery dissection is indeed found, the patient should be managed by either neurosurgery or neurology, and treated with either anticoagulation (heparin, enoxaparin) or antiplatelet therapy (aspirin, clopidogrel).
Partial/Complete CN-III Palsy
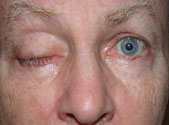
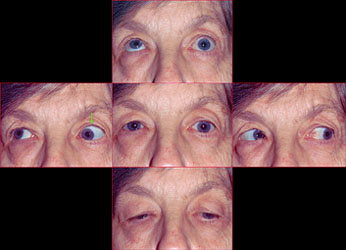
The ptosis of CN-III (oculomotor nerve) palsy is typically accompanied by an external ophthalmoplegia. The patient may not complain of diplopia if the ptosis is severe enough to occlude the visual axis.
As already noted, extraocular motility and pupillary size/reactivity must be checked and documented in all patients presenting with ptosis. The management of CN-III palsy is typically divided into four rubrics:
|
• Pupil-sparing, complete CN-III palsy. In patients over 55 years, the etiology is most likely microvascular and occurs in vasculopaths (hypertensives, diabetics). Patients are typically followed daily for one week to assure the pupil remains uninvolved, although many experts feel that if the palsy is complete and no anisocoria is noted on presentation, such daily follow-up is unnecessary. Neuroimaging is reserved for those cases that develop pupillary involvement or fail to resolve over three months. Note that in all younger patients, timely radiographic investigation is indicated even in the clinical context of “pupil-sparing, complete CN-III palsy.” One common misconception is that “aneurysmal thirds” always present with headache and “microvascular thirds” do not. In fact, the presence or absence of headache is not a helpful sign in distinguishing the etiology of CN-III palsy.
|
• Pupil-involving CN-III palsy. This is a true ophthalmic and neurosurgical emergency. All patients should undergo immediate neuroimaging with MRI/MRA or CT/CTA. The final decision regarding the type of imaging is dependent on the preference of the neuroradiologist; age and gender of the patient (CT/CTA should be avoided in young children and pregnant women); other patient comorbidities; and the availability of the imaging. If any significant delay in imaging is anticipated, an emergent CT of the head should be performed to rule out subarachnoid hemorrhage; if present, intracranial hemorrhage greatly increases morbidity and mortality and in such cases, immediate angiography should be performed (See Figure 3).
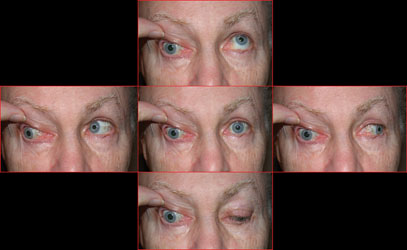
• Aberrant CN-III palsy. All patients should undergo MRI as a routine, outpatient test (See Figure 4). Microvascular CN-III palsy rarely (for all intents and purposes, never) results in aberrant regeneration; one should never assume that this is the etiology.
Another simpler, albeit less academic, management schema is to simply image all patients presenting with CN-III palsy of any type with MRI/MRA or CT/CTA immediately. Many may disagree and argue that this is defensive medicine. On the other hand, an “aneurysmal third” is a life-threatening emergency that has an excellent prognosis if managed in a timely fashion. It is a diagnosis that cannot be missed. That fact coupled with the advances in relatively noninvasive and readily available neuroimaging, as well has the relative inexperience of most non-neuro-ophthalmologists with CN-III palsy, should greatly decrease the threshold for urgently imaging all patients presenting with acute CN-III dysfunction. REVIEW
Dr. Bilyk is an attending surgeon in the Oculoplastic and Orbital Surgery Service at the Wills Eye Institute, and an associate professor of ophthalmology at Jefferson Medical College. Contact him at Neuro-Ophthalmologic Associates (215) 825-4796.