While there are numerous filler types available to the clinician, hyaluronic acid is the most commonly utilized today. Hyaluronic acid fillers not only replace the volume loss that is experienced with aging but also stimulate collagen production. A study by Taihao Quan, MD, PhD, and colleagues demonstrated increased fibroblast proliferation, expanded vasculature and increased epidermal thickness when hyaluronic acid was injected into the skin of patients older than 70 years of age. They hypothesized that fillers stiffen the extracellular matrix, induce fibroblast elongation and activation, and upregulate the transforming growth factor-beta pathway, leading to collagen synthesis.3
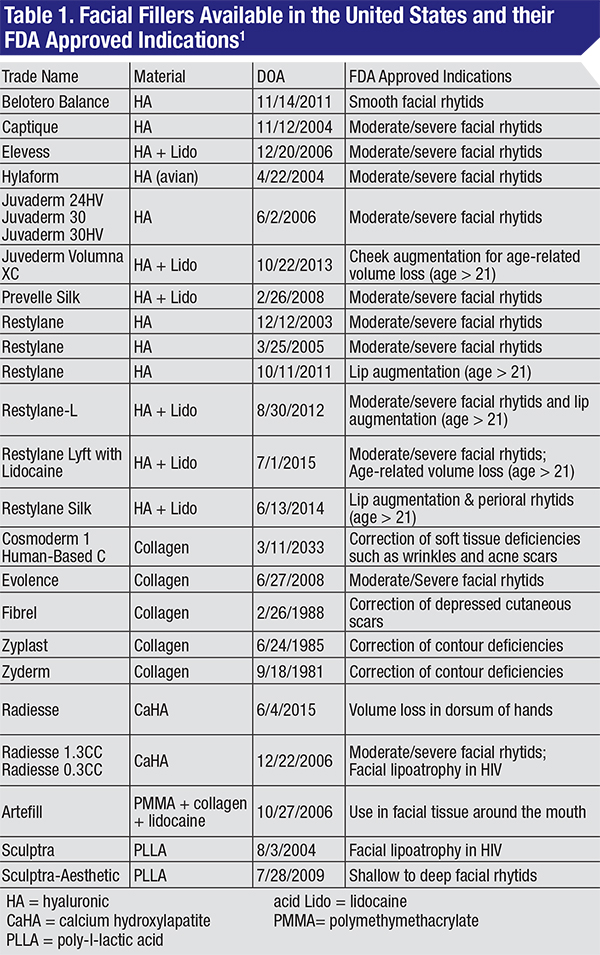
Currently, more than 20 dermal fillers have FDA approval for use in the United States. Filler indications range from lip and cheek augmentation to treatment of moderate to severe facial rhytids (See Table 1).1 Here, we will discuss the array of materials approved for use as soft-tissue filler in the United States, their ndications and known associated complications.
Types of Filler
 |  |
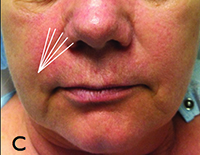 | 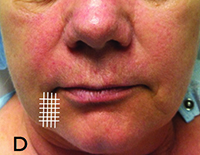 |
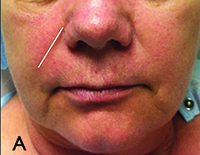
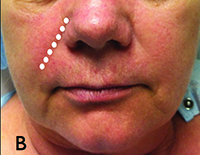
| Figure 1. Methods for filler injection. A. Linear threading; B. Serial puncture; C. Radial fanning; D. Cross hatching. | |
Absorbable Fillers
• Collagen. Collagen is a protein that is naturally occurring throughout the human body and is found in the skin, bones, tendons and numerous other tissues. Collagen was the earliest filler material used for the treatment of rhytids and is primarily derived from purified human or bovine collagen products. Collagen fillers have the shortest length of effect of all the filler materials4-6 and their effects generally last for about three to four months.7
• Hyaluronic acid. Hyaluronic acid is a polysaccharide that is naturally occurring throughout the human body and is found in numerous tissues, including the skin, synovial fluid, certain types of cartilage and other connective tissues. Hyaluronic acid fillers are derived from purified bacteria and avian (rooster combs) products. Hyaluronic acid is an excellent filler choice because it is biodegradable, biocompatible and non-immunogenic (i.e., has a very low potential for stimulating allergic reaction). It has excellent viscoelastic and hygroscopic properties, which create good volume expansion for the effacement of facial rhytids.8
• Poly-l-lactic acid. PLLA is a biodegradable and biocompatible synthetic polymer that has widespread medical applications as absorbable sutures, bone screws and soft-tissue fillers.7 As a filler, PLLA is reconstituted with sterile water into a hydrogel with a methylcellulose carrier. PLLA achieves its effects by causing a foreign-body reaction that stimulates collagen formation and dermal fibrosis.8 In order to achieve the desired amount of volume replacement, PLLA is injected in multiple treatment sessions over a period of months.
Effects may not be seen for several weeks to months but can last for years, with periodic touch-ups.
• Calcium hydroxylapatite. CaHA is a biocompatible and nonimmunogenic material that is naturally occurring in human bones and teeth. CaHA fillers are synthetic bone microspheres suspended in a carboxymethylcellulose carrier gel. Its effects last approximately 18 months.7 It should be noted that this filler type is visible on radiographs and may obscure underlying structures on radiographic images.
Non-absorbable Filler
• Polymethylmethacrylate. PMMA is a non-biodegradable, biocompatible synthetic material that is used in bone cement, intraocular lens implants and soft tissue fillers. As a dermal filler, PMMA microspheres are suspended in a bovine-based collagen and lidocaine solution and injected over a period of several months. PMMA microspheres are permanent and not absorbed by the body.
Indications & Uses
The main indications for injectable fillers include the filling of rhytids and folds, and the correction of soft-tissue loss due to disease or age. The FDA has approved most fillers for the treatment of moderate to severe wrinkles and localized fat loss (lipoatrophy) in the face. Restylane, Restylane-L and Restylane Silk have also been approve dfor lip augmentation in patients over 21 years of age. As of October 2013, Juvederm Volumna XC received approval for augmentation of the cheeks in patients over 21 years of age (See Table 1). All other uses of fillers are considered off-label and should be disclosed as such to patients.1,7,9
Commonly utilized off-label applications include volume replacement and enhancement procedures such as cheek and chin augmentation, lip enhancement, hand rejuvenation, tear trough obliteration, nose reshaping, mid-facial volumization and correction of facial asymmetry.
Relative Contraindications
The FDA recommends that patients with the following conditions avoid the use of facial fillers:9
• history of bleeding disorders;
• allergy to eggs or cow collagen;
• any history of allergy causing anaphylactic shock;
• allergy to lidocaine;
• history of or predilection for keloid formation; and
• actively inflamed or infected skin.
Injection Methods
Numerous methods have been discussed in regards to injecting dermal fillers. The depth of the injection depends on the properties of each product and the desired outcome.
 |
• Linear threading. In linear threading, as the needle is withdrawn, a tunnel of filler is injected to efface the wrinkle. This technique is commonly used to address isolated creases such as nasolabial folds or marionette lines. In this technique superficial injection of the filler can occur toward the end of the needle withdrawal process, resulting in the Tyndall effect (See Figure 1A).
• Serial puncture. In this technique serial injections are made along the length of a wrinkle where small aliquots of filler are deposited. There is potential for more bruising with this technique (See Figure 1B).
• Radial fanning. The goal of radial fanning is to fill the problem area with minimal skin punctures in hopes of decreasing bruising. A needle is inserted in the desired region and a tunnel of filler is injected as the needle is withdrawn; however, before the needle is completely removed from the skin, it is redirected into a different radial plane and more filler is injected until the desired outcome is achieved (See Figure 1C).
• Cross hatching. Cross hatching builds on linear filling where a series of parallel tunnels of filler is injected. Then perpendicular tunnels are injected to create a cross hatching. This technique is meant for filling in broader areas (See Figure 1D).
Complications
Several complications have been reported with the injection of soft-tissue fillers. Complications from use of non-absorbable fillers (PMMA) are more difficult to manage: As they are not absorbed they may need to be excised. This may result in unwanted scarring and ultimately poor cosmetic results. As a general rule, hyaulonic acid filler complications may be reversed using hyaluronidase, providing a distinct advantage for the clinician in the management of potential complications.10
• Bruising. The most common complication seen with fillers is bruising of the injection site. This can occur regardless of the technique used. However, the following steps can be taken to minimize or prevent bruising:
• Instruct patients to avoid using blood thinners (aspirin, warfarin, clopidogrel, dabigatran, nonsteroidal anti-inflammatory drugs, fish oil, vitamin E, garlic, gingko or ginseng) for one week prior to injection.
• Use small-gauge needles and blunt cannulas.
• Minimize the number of puncture sites.
• if bruising is noted or suspected at the time of injection, immediately hold pressure over the area and apply an ice pack.
• Advise patients to avoid activities that can raise their blood pressure or put strain on their head and face for 24 hours after injection (e.g., exercise and activities that cause valsalva).
• Advise patients to keep their head elevated for 24 hours after injection.
• Over-filling. Overfilling may result from injecting too much filler into a small area and may create lumps, nodules or asymmetry. Treatment includes: hyaluronic acid—injection of hyaluronidase into area of over-filling; other fillers—incision and drainage of the filler; and large volume nodules—injection of local anesthetic and aspiration of filler with a larger-bore needle.10 Use caution when planning for correction of multiple nodules within vital anatomical structures.
• Under-filling. Under-filling may result from injecting too little filler into a rhytid, and is treated by injecting more filler in the area of under-filling.
• Granulomatous inflammation. Granulomatous inflammation may result from any type of filler. True granulomas from fillers will likely involve multiple sites of injections. Solitary nodules are likely not true granulomatous reactions. Treatment may include graduated injections over period of weeks to months with one of the anti-inflammatory medications, kenalog, triamcinolone or 5-fluorouracil.10
• Tyndall effect. The Tyndall effect results from injecting hyaluronidase fillers too superficially, which places the filler close to the surface of the skin. It causes a bluish discoloration to the overlying skin, which looks like a deep bruise. This complication will not improve until the filler is removed. Treatment entails injection of 15 to 50 IU of hyaluronidase, and gentle massage by rolling a cotton-tipped applicator over the area to disperse the hyaluronidase.10
• Infection at injection site. This is an uncommon complication with fillers. It may be caused by bacterial, viral or Candida species. Herpes simplex is the most common viral infection to spread to an injection site, and is more common in patients with a strong history of cold sores. Consider pretreating with acyclovir, valacyclovir or famciclovir to reduce the risk of complication. Maintain a low threshold for complete ophthalmologic evaluation if there is any concern for ocular involvement of herpes simplex virus.10
In cases of abscess formation, treatment involves incision and drainage ±oral antibiotics to cover common skin flora, gram positives if there is concern for cellulitis. Hyaluronidase should always be avoided any time there is suspicion for infection, as it may allow for further spreading of infection through the surrounding soft tissue.
• Biofilms. Biofilms may result from any implantable device or foreign material placed within the body. They allow bacteria to lie dormant for a long period, then awaken later in time to cause granulomatous inflammation, abscess formation or cellulitis. Treatment includes:
• Permanent fillers (PMMA)—incision and removal of filler. This is why caution must be used when considering permanent fillers for use in vital anatomical structures such as lips and eyelids.
• Hyaluronic acid—injection of hyaluronidase.
• Broad-spectrum oral antibiotics (fluoroquinolones or macrolides).
• Avoid all steroids and NSAIDs.
If infection persists after above treatment, consider laser lysis or incision and drainage.1,10,11
• Vascular necrosis.12 Vascular necrosis is an extremely rare complication from filler use but still must be discussed with patients. The risk for all vascular complications increases with deeper large bolus filler injections. It results from accidental intravascular injection of filler and may lead to local or distant ischemic necrosis of soft tissue. The local injection site necrosis is most commonly seen in the glabella, a potential vascular watershed region.
Clinical signs and symptoms include blanching; mottled discoloration of skin (livedo reticularis); pain; dusky skin discoloration; and sluggish or absent capillary refill. Immediate treatment is critical for recovery of vascular supply and includes:
• injection of hyaluronidase directly into the ischemic tissue (works if injected filler is HA);
• oral aspirin;
• topical nitropaste over ischemic tissue;
• warm compress and massage of the affected tissue; and
• hyperbaric oxygen therapy, which may be considered in persistent cases failing initial treatment with above.
• Central retinal artery occlusion. CRAO is an extremely rare complication from filler use but still must be discussed with patients.12 It presents with sudden visual field defect or vision loss. Funduscopy is warranted and may show signs of CRAO.
Treatment includes ocular massage; hyperventilation; oral aspirin; and hyperbaric oxygen therapy. Treatment is widely ineffective for CRAO related to filler embolus. REVIEW
Drs. Milam, Zhang and Barahini are at the Vanderbilt Eye Institute, Vanderbilt University Medical Center. Contact Dr. Milam at 4505 Georgia Ave. Nashville, Tenn. 37209. Phone: (704) 929-8811; fax: (615) 936-4979; Email: ronald.milam@vanderbilt.edu.
1. Soft tissue fillers approved by the center for devices and radiological health. U.S. Food and Drug Administration website. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/Cos meticDevices/WrinkleFillers/ucm227749.htm. Updated 2015. Accessed 08/30/2015.
2. 2013 Plastic Surgery Statistics Report. American Society of Plastic Surgeons.
3. Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol 2013;133(3):658-667.
4. Narins RS, Brandt F, Leyden J, Lorenc ZP, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg 2003;29(6):588-595.
5. Lindqvist C, Tveten S, Bondevik BE, Fagrell D. A randomized, evaluator-blind, multicenter comparison of the efficacy and tolerability of perlane versus zyplast in the correction of nasolabial folds. Plast Reconstr Surg 2005;115(1):282-289.
6. Baumann LS, Shamban AT, Lupo MP, et al. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: A multicenter, double-masked, randomized, within-subject study. Dermatol Surg 2007;33 Suppl 2:S128-35.
7. Soft tissue fillers (dermal fillers). U.S. Food and Drug Administration website. http://www.fda.gov/medicaldevices/productsand medicalprocedures/cosmeticdevices/wrinklefillers/default.htm. Updated 2015. Accessed 08/30/2015.
8. Kontis TC. Contemporary review of injectable facial fillers. JAMA Facial Plast Surg 2013;15(1):58-64. .
9. Filling in wrinkles safely. FDA Consumer Updates. Aug 2014.
10. DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J 2013;33(4):561-575.
11. Dayan SH, Arkins JP, Brindise R. Soft tissue fillers and biofilms. Facial Plast Surg 2011;27(1):23-28.
12. DeLorenzi C. Complications of injectable fillers, part 2: Vascular complications. Aesthet Surg J 2014;34(4):584-600.



