The prevalence of myopia is increasing. Nearly half of the world’s population is predicted to suffer from myopia by 2050, with 10 percent of the population being high myopes.1 (High myopia is generally defined as a refractive error of more than -6 D or an axial length longer than 26 or 26.5 mm, although some studies define high myopia as a refractive error of at least -5 D.)2,3 In addition to the obvious vision difficulties posed by myopia, high myopia can also be associated with the vision-threatening ocular structural changes of pathologic myopia. In this article, we’ll detail the most effective way to diagnose and manage a patient with this condition.
Pathologic Myopia Overview
Pathologic myopia (myopic degeneration) is defined as the presence of structural changes in the posterior segment owing to an increased axial elongation.4 Although the cut-off values of the refractive error and axial length aren’t specified in the definition, pathologic myopia is usually associated with high myopia.
In the following sections, we’ll delve into each of the vision-threatening conditions that are known to occur in eyes with pathologic myopia, including myopic choroidal neovascularization, myopic subretinal hemorrhage, myopic choroidal atrophy, dome-shaped macula, posterior staphyloma, myopic traction maculopathy and macular hole retinal detachment.
Myopic CNV
Myopic CNV is one of the most common vision-threatening complications in patients with pathologic myopia.5 It affects 5 to 11 percent of patients with pathologic myopia and is bilateral in approximately 15 percent.6 Patients with myopic CNV present with metamorphopsia (distortion of vision) and/or visual impairment similar to those with age-related macular degeneration. Ocular risk factors include lacquer cracks (fissures in the retinal pigment epithelium–Bruch’s membrane–choriocapillaris complex), choroidal thinning, impaired choroidal circulation and patchy retinal atrophy in the posterior pole.7 Incidence of myopic CNV is higher in eyes with lacquer cracks (29 percent) than in eyes with other types of myopic maculopathy.8
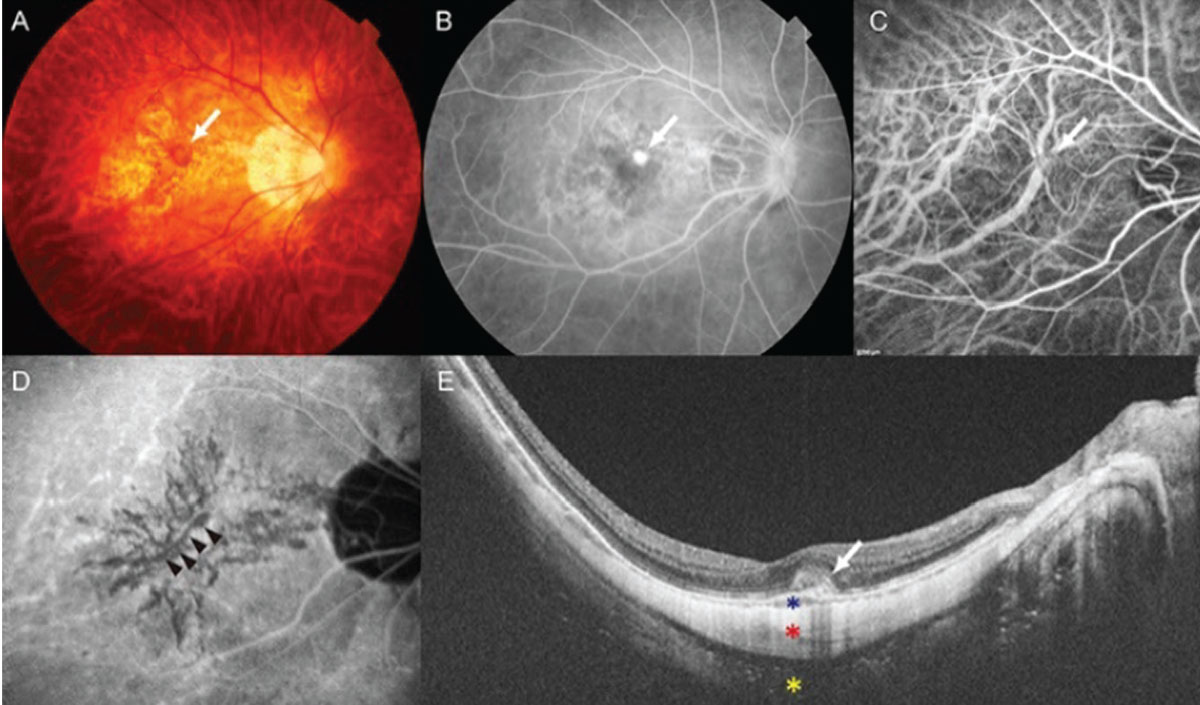 |
| Figure 1. A 65-year-old female presented with a visual acuity of 20/30. The axial length was 29.5 mm. (A) Myopic choroidal neovascularization with hemorrhage (arrow). (B) Fluorescein angiography showing leakage (arrow). (C) Early-phase indocyanine green angiography revealing CNV (arrow). (D) Late-phase indocyanine angiography revealing lacquer cracks, a known risk factor for myopic CNV (arrows). (E) Swept-source optical coherence tomography shows a dome-shaped hyper-reflective lesion with subretinal fluid (white arrow). Asterisks indicate choroid (blue), sclera (red), and orbital fat (yellow). |
On examination, the CNV lesion often manifests as a light-colored lesion with a dark, hyperpigmented rim, with or without subretinal hemorrhage, and sometimes exudates (Figure 1). The diagnosis can be confirmed using several imaging techniques, including fluorescein angiography, indocyanine green angiography, spectral-domain optical coherence tomography, swept-source OCT and OCT angiography. Among these, FA conventionally has been the gold standard, particularly for the initial diagnosis of CNV. FA reveals hyperfluorescence in the early phase and dye leakage in the later phases, based on the CNV activity. However, because FA is an invasive diagnostic exam with potential side effects, SD-OCT is commonly used during follow-ups (or even for initial diagnosis) since it is a non-invasive technique for assessing the activity of the myopic CNV.
Active myopic CNV often reveals a dome-shaped hyperreflective elevation above the RPE, accompanied by subretinal fluid. When the activity of myopic CNV is uncertain, with minimal subretinal fluid on SD-OCT, SS-OCT may be helpful for the detection of very small CNV and subtle fluid (Figure 2). SS-OCT uses longer wavelengths and can penetrate deeper into the posterior segment structures. A relatively thicker sclera and a thinner choroid are some biological indicators for myopic CNV on SS-OCT.9 Early-phase ICGA provides information regarding choroidal circulation, and late-phase ICGA is useful for visualizing lacquer crack formation, which is responsible for the development of myopic CNV in many cases.10
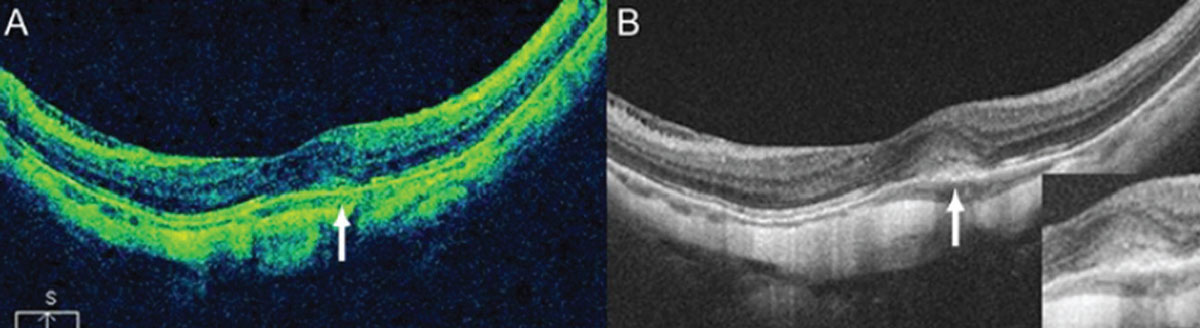 |
Figure 2. Although SD-OCT (A) is useful in detecting myopic CNV in most cases, SS-OCT (B) can more clearly detect CNV and subtle subretinal fluid, and better image the choroid. |
OCTA is a non-invasive method of detecting myopic CNV, and is particularly useful in patients who are allergic to fluorescein dyes (Figure 3). A recent study demonstrated that the acquisition rate of clear OCTA images in myopic CNV was as high as 76 percent.11 The evaluation of the activity of myopic CNV with OCTA has also been attempted by assessing vascular branching and anastomoses of the CNV lesion.11 However, evaluating the activity of the CNV lesion with OCTA alone is usually difficult because CNV lesions persist even after anti-vascular endothelial growth factor treatment.
The long-term outcome of myopic CNV is very poor if left untreated (visual acuity worsens to 20/200 or less within five years).12 Intravitreal anti-VEGF injection is the current first-line treatment for myopic CNV.5 Ranibizumab (Lucentis; Genentech) is the only FDA-approved medication; however, off-label use of bevacizumab has also been effective. Various clinical trials, such as MYRROR, RADIANCE, BRILLIANCE and SHINY, have demonstrated the safety and efficacy of anti-VEGF treatment for myopic CNV.13 Early initiation of treatment is strongly recommended to achieve optimal outcomes.14
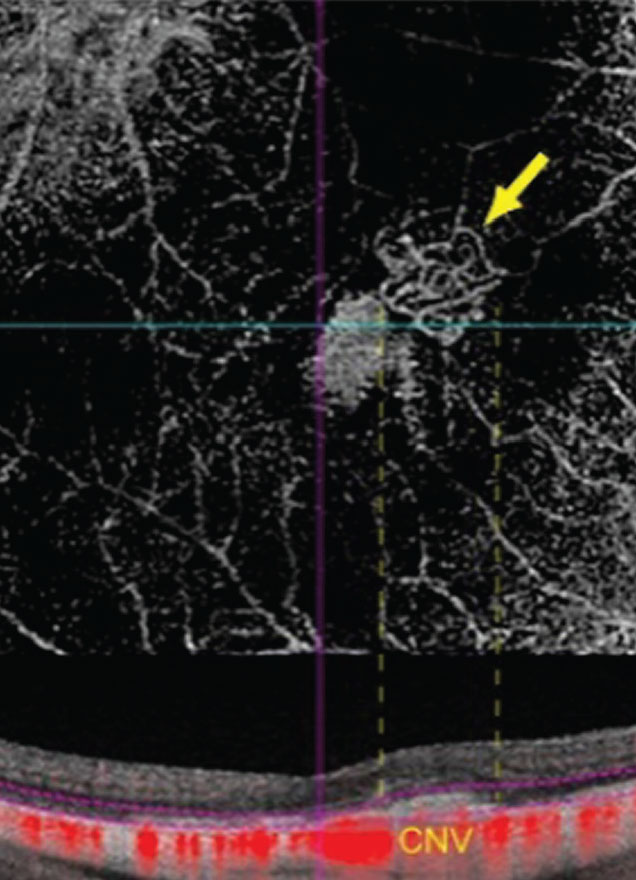 |
| Figure 3. Non-invasive visualization of myopic CNV is possible with OCT angiography. |
The treatment schedule will depend on disease activity. Compared to neovascular age-related macular degeneration, myopic CNV tends to regress more quickly, requiring fewer injections.15,16 One study even showed that 67 percent of patients with myopic CNV (34/51 eyes) received just one injection during five years of follow-up.17 However, some older patients may develop larger CNVs that resemble neovascular AMD, and may require multiple injections to achieve resolution.
The timing of and decision about retreatment for recurrent CNV can be challenging due to the subtlety of the imaging findings in myopic CNV. Visual impairment, subjective symptoms and OCT imaging should all be considered when evaluating for recurrences. Most recurrences occur within the first year following the onset of disease. A subsequent increase in the subfoveal choroidal thickness after an initial decrease following anti-VEGF therapy has been associated with a higher chance of CNV recurrence.18
Myopic Subretinal Hemorrhage
Myopic subretinal hemorrhage is often caused by lacquer crack formation in pathologic myopia.19 It has a prevalence of nearly 3 percent in highly myopic eyes. Patients often present with symptoms similar to those of myopic CNV. On examination, subretinal hemorrhage is observed at the macula, similar to myopic CNV. However, FA shows blocked hypofluorescence, associated with hemorrhage, but without hyperfluorescent leakage (Figure 4). SD-OCT may show subretinal fluid and projection of the hemorrhage within the retina, along with the Henle’s fiber layer in some cases.19 Late-phase ICGA is useful in visualizing the lacquer cracks; OCTA is useful for confirming the absence of myopic CNV.
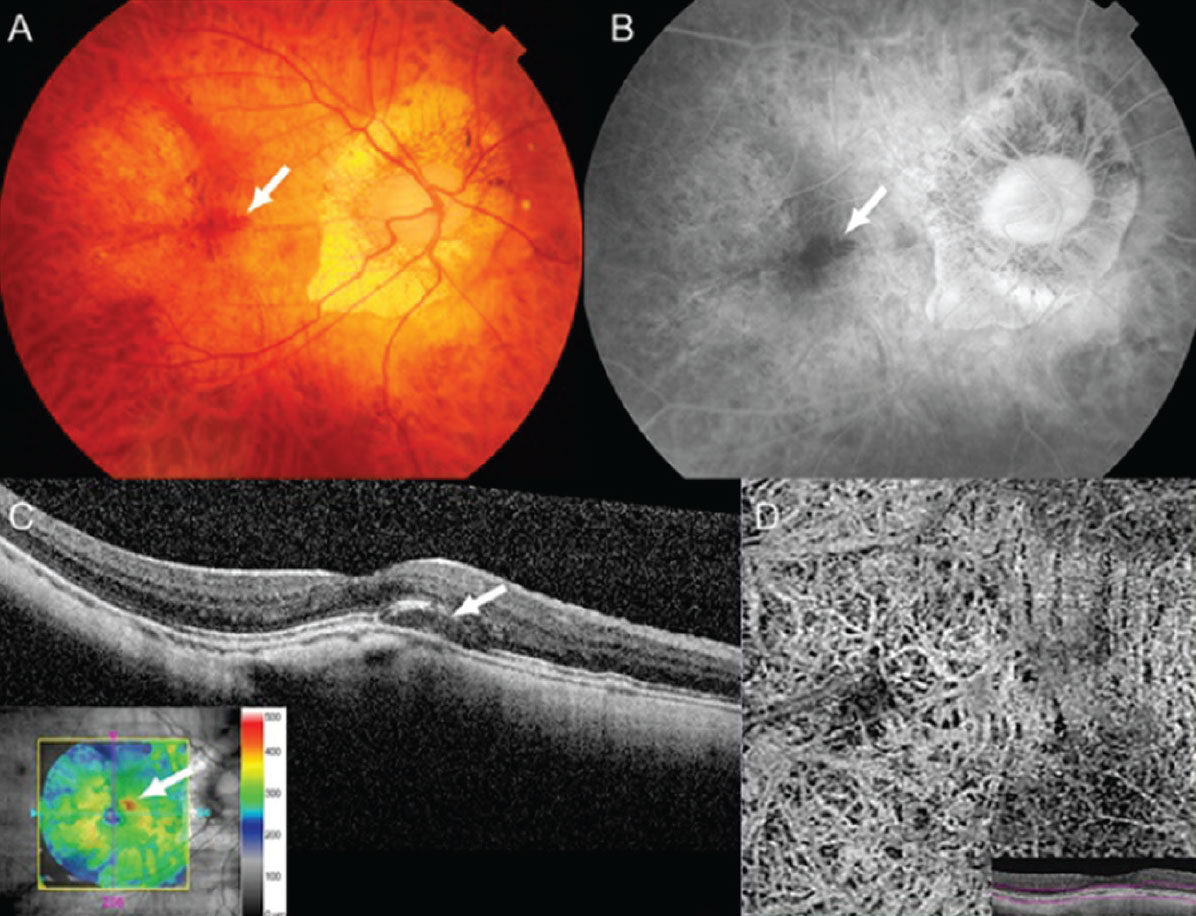 |
| Figure 4. A 61-year-old female presented with blurred vision. (A) Fundus photograph showing submacular hemorrhage (white arrow). (B) Fluorescein angiography showing hypofluorescence associated with hemorrhage but without leakage (white arrow). (C) Swept-source optical coherence tomography (SS-OCT) showing elevation of the retina due to subretinal hemorrhage and fluid (white arrow). (D) OCT angiography shows an absence of myopic CNV. |
The distinction between myopic CNV and hemorrhage from lacquer cracks is very important to make, because the treatment is different. Anti-VEGF treatment is not indicated in myopic subretinal hemorrhage without CNV. These patients have a more favorable visual prognosis than those with myopic CNV. Indeed, patients gradually recover vision without treatment, since the hemorrhage spontaneously resorbs. Some patients may have persistent visual impairment owing to damage to the photoreceptor cells caused by hemorrhage.20 However, intervention is not required for this condition.
Myopic Choroidal Atrophy
Chorioretinal atrophy is a common feature of pathological myopia. Atrophy is usually progressive, associated with age, axial length, myopic CNV and posterior staphyloma, and can occur in different patterns. The most recent classification (META-PM classification) categorizes myopic macular lesions into five categories: no myopic retinal lesions (category 0); tessellated fundus only (category 1); diffuse chorioretinal atrophy (category 2); patchy chorioretinal atrophy (category 3); and macular atrophy (category 4).21 Three additional features—lacquer cracks, myopic CNV and Fuchs’ spots—were added to these categories as “plus signs.” Based on this classification, pathologic myopia is defined as equal to or greater than category 2, or the presence of a plus lesion or posterior staphyloma.
Myopic CNV is associated with further progression of chorioretinal atrophy (Figure 5). Despite the stabilization of myopic CNV with anti-VEGF treatment, more than 90 percent of eyes experience a significant decline in vision during five to 10 years of follow-up, mainly owing to the development and progression of patchy retinal atrophy around regressed CNV tissue.12 A study using SS-OCTA revealed that CNV-related macular atrophy wasn’t simply chorioretinal atrophy but was an enlarged break in the Bruch’s membrane around the myopic CNV.21 Progressive chorioretinal atrophy could result in significant vision loss. Currently, no treatment exists for the slowing or recovery of chorioretinal atrophy, similar to atrophy following neovascular AMD. Since myopic chorioretinal atrophy is a leading cause of blindness in many countries, the development of a treatment is strongly desired.
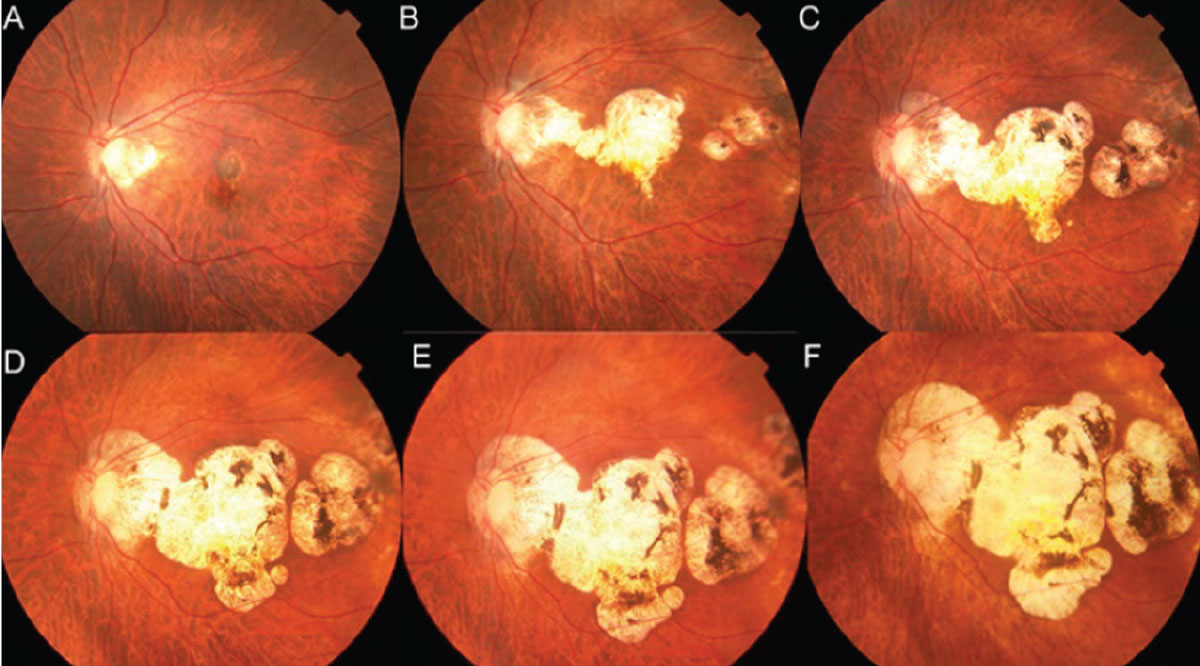 |
| Figure 5. A 57-year-old female presented with myopic choroidal neovascularization. The axial length was 27.6 mm. During 20 years of follow-up, chorioretinal atrophy gradually progressed (A–F). Visual acuity decreased from 20/400 (A) to hand motions (F). |
Dome-shaped Macula
Dome-shaped macula is an inward bulge of the macula within the chorioretinal posterior concavity of the eye that’s best visualized with OCT imaging (Figure 6).22 Three dimensional-magnetic resonance imaging (3D-MRI) has demonstrated morphological changes in the entire posterior pole, with a band-shaped inward convexity that extends horizontally from the optic disc through the fovea.23 There are certain controversies regarding the choroidal thickness beneath the DSM. However, the central choroid seems to be thicker than the choroid in the surrounding staphyloma, and a relatively thicker sclera exists at the bulge apex.24
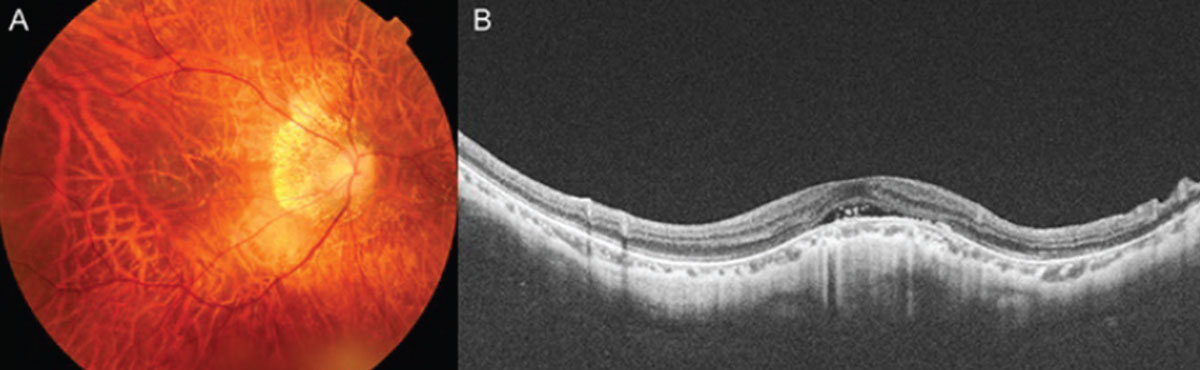 |
| Figure 6. A case of dome-shaped macula. A 54-year-old female presented with visual acuity of 20/50. Swept-source optical coherence tomography shows subretinal fluid at the dome apex. This fluid is not from choroidal neovascular membranes, and doesn’t respond to anti-VEGF treatment. |
Isolated DSM can be associated with subretinal fluid, pigment epithelium detachment, CNV and polypoidal choroidal vasculopathy at the dome apex. Anti-VEGF isn’t indicated for subretinal fluid from DSM alone, without CNV lesions. However, CNV or PCV should be promptly treated with anti-VEGF injections. The post-hoc analysis of the RADIANCE study (a randomized, controlled study of ranibizumab in patients with CNV secondary to pathologic myopia) confirmed that CNV with or without dome-shaped macula equally responds to anti-VEGF therapy, and the presence of dome-shaped macula doesn’t alter the visual prognosis following ranibizumab treatment.25
Posterior Staphyloma Issues
Posterior staphyloma is an outpouching of the eye wall with a radius of curvature less than the surrounding curvature of the eye, and it’s a hallmark of pathologic myopia.21 Nearly half of patients with pathologic myopia have staphylomas, and it’s hypothesized that tissue loss and changes in the collagen fibrils cause scleral weakness. Posterior staphyloma was originally classified into 10 types; however, using 3D-MRI and widefield fundus imaging, it’s recently been reclassified into six categories: the wide macular (most predominant type, 74 percent of the eyes with staphyloma); the narrow macular; peripapillary; nasal; inferior; and other configurations.26 There’s no widely accepted treatment for posterior staphyloma; however, scleral regeneration and scleral collagen cross-linking are being investigated.
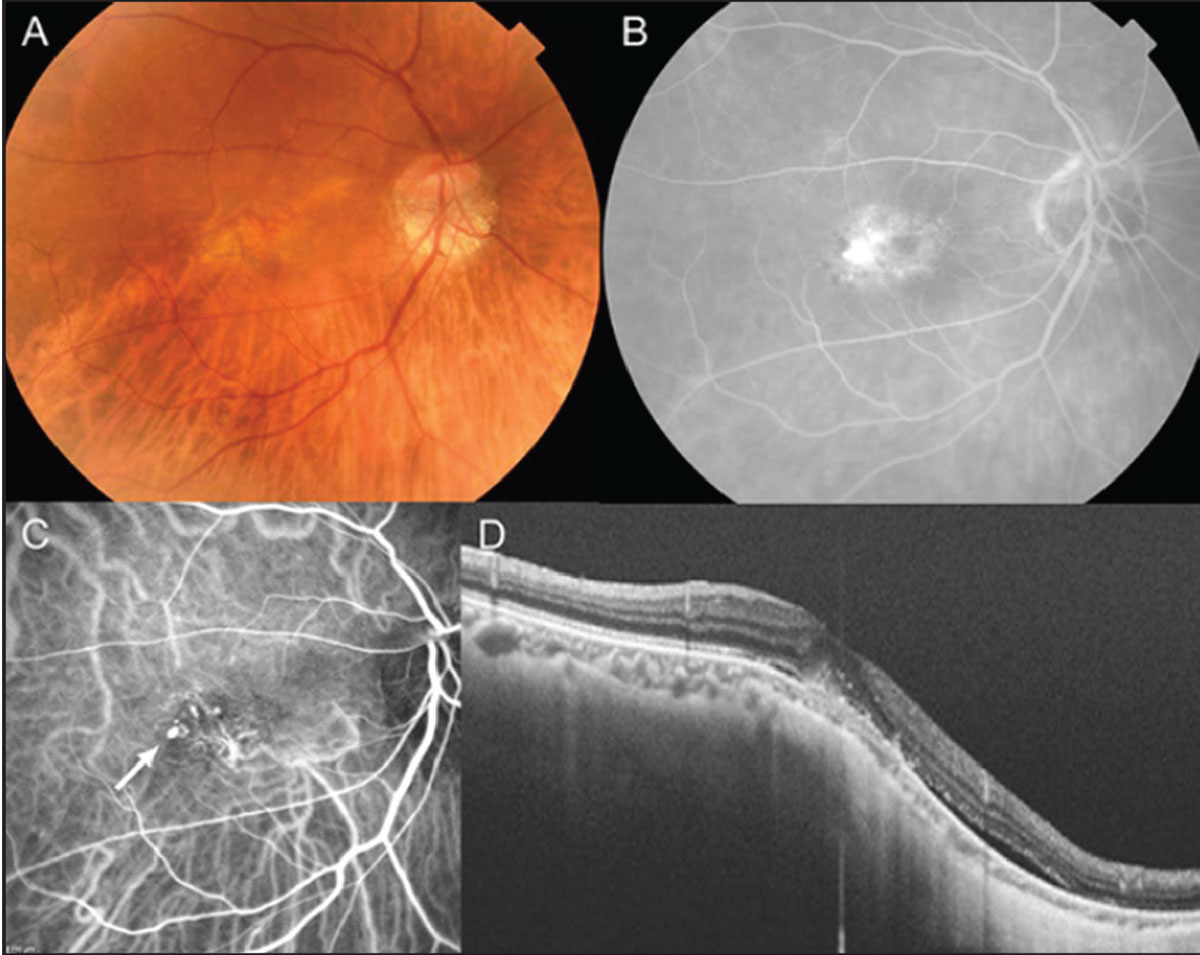 |
| Figure 7. A 72-year-old female presented with visual acuity of 20/30. (A) Fundus photograph showing that the upper border of the inferior posterior staphyloma lies across the macula. (B) Fluorescein angiography showing leakage. (C) Indocyanine green angiography showing polypoidal choroidal vasculopathy in the macula (white arrow). (D) Swept-source optical coherence tomography showing shallow pigment epithelial detachment and subretinal fluid. |
Inferior posterior staphyloma is a subtype of posterior staphyloma and is commonly associated with tilted disc syndrome (Figure 7). When the upper border of the inferior posterior staphyloma lies across the macula, macular complications may develop, resulting in visual impairment. Macular complications include macular atrophy, subretinal fluid, CNV and PCV. Similar to the treatment for complications associated with dome-shaped macula, CNV and PCV can be treated using anti-VEGF.27
Myopic Traction Maculopathy
Myopic traction maculopathy is characterized by retinal thickening, retinoschisis, lamellar macular hole formation and foveal detachment associated with high myopia (Figure 8). Recently, ultra-widefield-SS-OCT revealed that MTM is present within the area of the staphylomas, and paravascular vitreal adhesions could play an important role in the development of MTM.28,29 During its natural course, MTM could progress to a full-thickness macular hole and macular hole retinal detachment, owing to collective retinal traction from the adherent vitreous cortex, epiretinal membrane, internal limiting membrane, retinal vessels and posterior staphyloma.30 Therefore, release of the retinal traction by pars plana vitrectomy with internal limiting peeling effectively resolves MTM and prevents macular hole and macular hole retinal detachment. However, postoperative macular hole opening could occur in 5 to 20 percent of the eyes following vitrectomy with ILM peeling, owing to the extremely thin fovea in MTM. The fovea-sparing ILM peeling technique is effective in preventing macular hole development.31
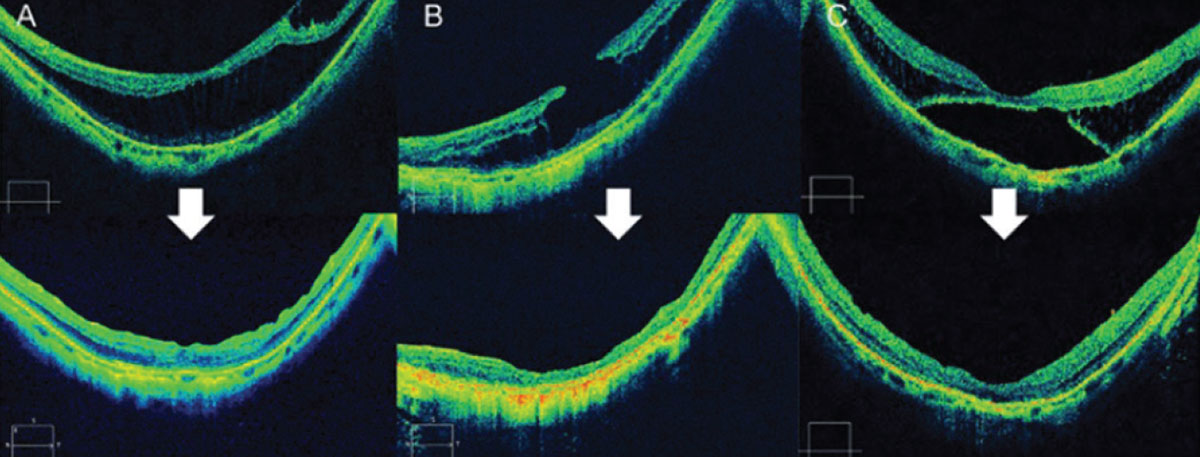 |
| Figure 8. (A) Spectral-domain optical coherence tomography image showing myopic traction maculopathy (retinoschisis type) in a 65-year-old woman. Axial length was 28.1 mm, and visual acuity was 20/50. The MTM was resolved 12 months after surgery. Visual acuity improved to 20/30. (B) SD-OCT image showing MTM (lamellar macular hole type) in a 76-year-old woman. Axial length was 31.9 mm, and visual acuity was 20/60. The MTM resolved 12 months after surgery. Visual acuity remained stable. (C) SD-OCT image showing MTM (foveal detachment type) in a 76-year-old woman. The axial length was 27.3 mm, and visual acuity was 20/50. The MTM resolved 12 months after surgery. Visual acuity improved to 20/30. |
Early stages of MTM without foveal detachment are relatively stable and asymptomatic, and aren’t indicated for surgery. However, surgical intervention is ideal before macular hole formation, so the best timing for surgery is gauged by changes in OCT imaging and when vision starts to become affected.
Macular Hole Retinal Detachment
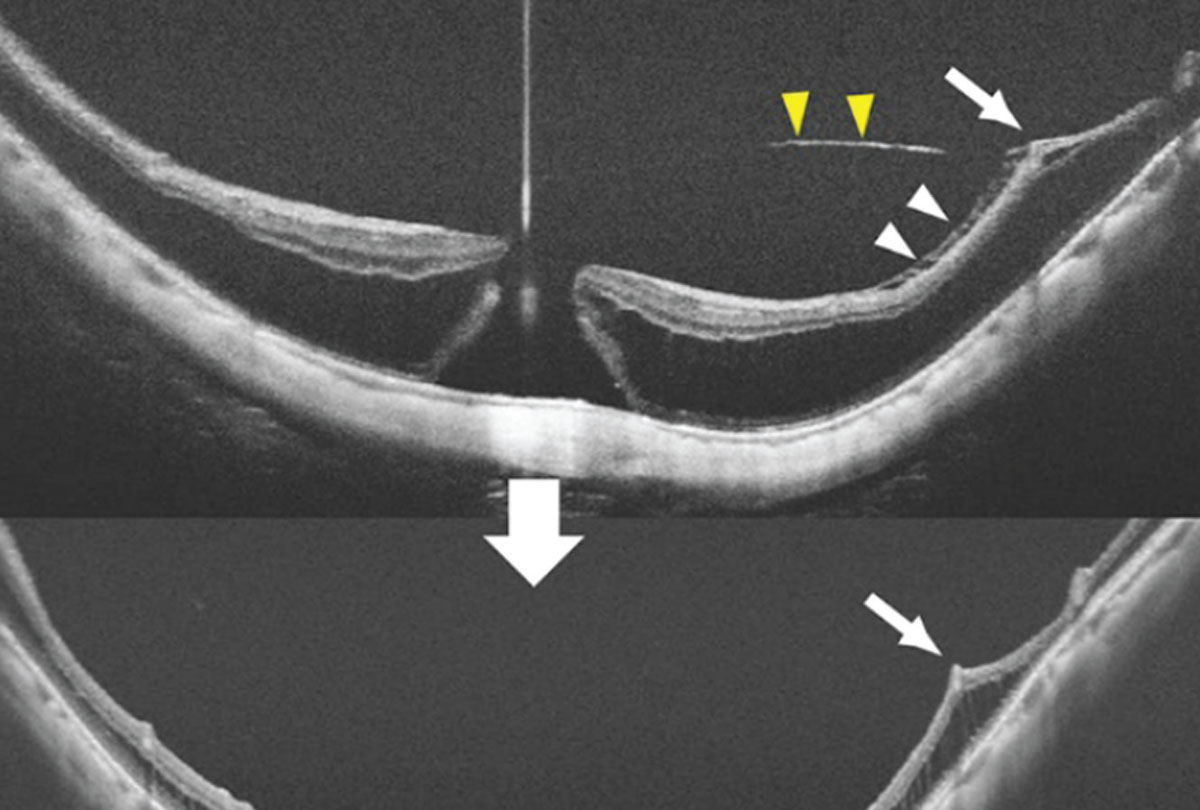 |
| Figure 9. A 62-year-old female with a myopic macular hole and retinoschisis. The posterior staphyloma and traction from the vitreous cortex (yellow arrowheads), internal limiting membrane (ILM, white arrowheads), and blood vessels (white arrow) contribute to this condition. Vitrectomy and an inverted ILM flap resulted in hole closure. |
Macular hole and macular hole retinal detachment are serious complications associated with pathologic myopia (Figure 9). The pathogenesis of macular hole involves vitreoretinal traction due to epiretinal membranes, remnants of the cortical vitreous, taut ILM and retinal vasculature adhesions. Axial elongation and posterior staphyloma contribute as well. This condition appears to be more common in Asian and female patients.32
Visual outcomes following vitrectomy for macular hole retinal detachment is usually poor (less than 20/200).33 The macular hole closure rate is approximately 40 percent following standard ILM peeling for macular hole retinal detachment, but the inverted ILM flap technique increases the rate of hole closure.34 Eliminating residual vitreous cortex and epiretinal membranes could decrease the likelihood of recurrent detachments. Macular buckling isn’t widely performed, but studies have demonstrated its efficacy.35
Other Complications
Glaucoma, cataract and rhegmatogenous retinal detachment occur more frequently in highly myopic eyes. Progressive axial length elongation has been reported in recent studies, even in adults,36 which could further promote chorioretinal atrophy.
In conclusion, various complications may occur in patients with pathologic myopia that can result in visual impairment. Recent advances in multimodal imaging for diagnosis and medical/surgical treatment have significantly improved our knowledge of the disease phenotype. However, further advancements are needed to prevent and treat conditions such as posterior staphyloma, persistent elongation of the axial length, and consequent chorioretinal atrophy. Prevention of myopia itself is also particularly important in preventing vision loss resulting from pathologic myopia-related complications.
Dr. Mahmoudzadeh is a retina research fellow at Wills Eye Hospital.
Dr. Patel is a vitreoretinal surgery fellow at Wills.
Dr. Wakabayashi is a former assistant professor of ophthalmology at Osaka University Graduate School of Medicine in Japan and a current retina post-doctoral research fellow at Wills.
1. Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123:5:1036–1042.
2. Flitcroft DI, He M, Jonas JB, et al. IMI – Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Investigative Ophthalmology & Visual Science 2019;60:3:M20–M30.
3. Ohno-Matsui K. What is the fundamental nature of pathologic myopia? Retina 2017;37:6:1043-1048.
4. Ohno-Matsui K, Lai TYY, Lai C-C, Cheung CMG. Updates of pathologic myopia. Prog Retin Eye Res 2016;52:156–187.
5. Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res 2018;63:92-106.
6. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: An evidence-based systematic review. Am J Ophthalmol 2014;157:1:9-25.
7. Ikuno Y, Jo Y, Hamasaki T, Tano Y. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci 2010;51:7:3721–3725.
8. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003;87:5:570–573.
9. Ruiz-Medrano J, Montero JA, Flores-Moreno I, et al. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res 2019;69:80–115.
10. Axer -Siegel Ruth, Cotlear D, Priel E, et al. Indocyanine green angiography in high myopia. Ophthalmic Surgery, Lasers and Imaging Retina 2004;35:2:139–145.
11. Li S, Sun L, Zhao X, et al. Assessing the activity of myopic choroidal neovascularization: Comparison between optical coherence tomography angiography and dye angiography. Retina 2020;40:9:1757–1764.
12. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: A 10-year follow-up. Ophthalmology 2003;110:7:1297–1305.
13. Ohno-Matsui K, Wu PC, Yamashiro K, et al. IMI pathologic myopia. Invest Ophthalmol Vis Sci 2021;62:5:5.
14. Cheung CMG, Ohno-Matsui K, Wong TY, et al. Influence of myopic macular degeneration severity on treatment outcomes with intravitreal aflibercept in the MYRROR study. Acta Ophthalmol 2019;97:5:e729–e735.
15. Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: The 12-Month Results from the REPAIR study. Ophthalmology 2013;120:9:1944-1945.
16. Cheung CMG, Arnold JJ, Holz FG, et al. Myopic choroidal neovascularization: Review, guidance, and consensus statement on management. Ophthalmology 2017;124:11:1690–1711.
17. Onishi Y, Yokoi T, Kasahara K, et al. Five-year outcomes of intravitreal ranibizumab for choroidal neovascularization in patients with pathologic myopia. Retina 2019;39:7:1289-1298.
18. Ahn SJ, Park KH, Woo SJ. Subfoveal choroidal thickness changes following anti-vascular endothelial growth factor therapy in myopic choroidal neovascularization. Invest Ophthalmol Vis Sci 2015;56:10:5794–5800.
19. Asai T, Ikuno Y, Nishida K. Macular microstructures and prognostic factors in myopic subretinal hemorrhages. Invest Ophthalmol Vis Sci 2014;55:1:226-32.
20. Goto S, Sayanagi K, Ikuno Y, et al. Comparison of visual prognoses between natural course of simple hemorrhage and choroidal neovascularization treated with intravitreal bevacizumab in highly myopic eyes: A 1-year follow-up. Retina 2015;35:3:429-34.
21. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 2015;159:5:877-883.
22. Gaucher D, Erginay A, Lecleire-Collet A, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol 2008;145:5:909–914.
23. Dai F, Li S, Wang Y, et al. Correlation between posterior staphyloma and dome-shaped macula in high myopic eyes. Retina 2020;40:11:2119–2126.
24. Imamura Y, Iida T, Maruko T, et al. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am J Ophthalmol 2011;151:2:297–302.
25. Ceklic L, Wolf-Schnurrbusch U, Gekkieva M, Wolf S. Visual acuity outcome in RADIANCE study patients with dome-shaped macular features. Ophthalmology 2014;121:11:2288–2289.
26. Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology 2014;121:9:1798–1809.
27. Doi A, Miyata M, Ooto S, et al. Long-term visual outcome in inferior posterior staphyloma and efficacy of treatment for complicated choroidal neovascularization. Am J Ophthalmol 2021;229:152-159.
28. Shinohara K. Tanaka N, Jonas JB, et al. Ultrawide-Field OCT to investigate relationships between myopic macular retinoschisis and posterior staphyloma. Ophthalmology 2018;125:10:1575-1586.
29. Takahashi H, Tanaka N, Shinohara K, et al. Importance of paravascular vitreal adhesions for development of myopic macular retinoschisis detected by ultra-widefield OCT. Ophthalmology 2021;128:2:256-265.
30. Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: Natural course and surgical outcome. Am J Ophthalmol 2007;143:3:455-462.
31. Shiraki N, Wakabayashi T, Ikuno Y, et al. Fovea-sparing versus standard internal limiting membrane peeling for myopic traction maculopathy: A study of 102 consecutive cases. Ophthalmol Retina 2020;4:12:1170-1180.
32. Nishimura A, Kimura M, Saito Y, Sugiyama K. Efficacy of primary silicone oil tamponade for the treatment of retinal detachment caused by macular hole in high myopia. Am J Ophthalmol 2011;151:1:148–155.
33. Nakanishi H, Kuriyama S, Saito I, et al. Prognostic factor analysis in pars plana vitrectomy for retinal detachment attributable to macular hole in high myopia: A multicenter study. Am J Ophthalmol 2008;146:2:198-204.
34. Chatziralli I, Machairoudia G, Kazantzis D, et al. Inverted internal limiting membrane flap technique for myopic macular hole: A meta-analysis. Surv Ophthalmol 2021;66:5:771-780.
35. Ripandelli G, Coppé AM, Fedeli R, et al. Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: A comparison of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology 2001;108:12:2258-2264.
36. Du R, Xie S, Igarashi-Yokoi T, et al. Continued increase of axial length and its risk factors in adults with high myopia. JAMA Ophthalmol 2021:e213303.



