Facial nerve palsy is a debilitating process that affects a person’s quality of life not only through functional impairment, but also from distorted self-perception and diminished psychosocial capacity. In this article, the first part in a two-part series on FNP, we review the anatomy of the facial nerve and etiologies and work up of FNP.
Background and Epidemiology
Persian physician Muhammad al-Razi wrote the earliest comprehensive descriptions of FNP in al-Hawi, a medical textbook published in the 10th century. Along with many other astute findings, he quoted Arab physician Ibn Batrigh’s description of FNP: “the patient’s face while smiling is crooked, and the eye on the affected side… always has tears running down it. The patient chews food on the unaffected side, they speak softly, and have a depressed mood.”1
Due to the extensive course of the facial nerve, there’s a wide spectrum of clinical presentations of FNP. The facial nerve affects some muscles of mastication, the auditory system, the lacrimal system, oral continence and salivation, speech and eyelid closure, which collectively form the crux of human communication and expression.2,3
The reported incidence of adult FNP is 17 to 35 cases per 100,000, and in neonates, 0.6-1.8 per 1,000 live births.2,4 There’s no sex, geographic or ethnic predilection.4
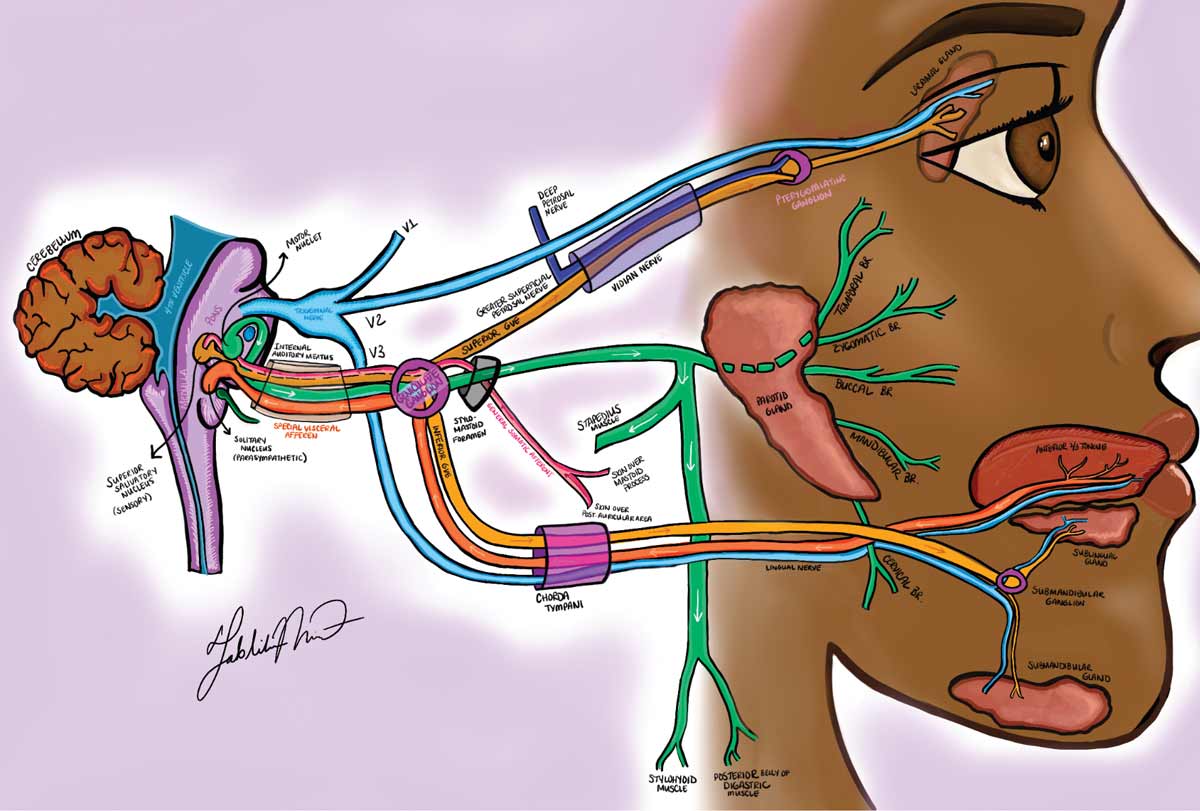 |
| Figure 1. The facial nerve pathway. Click image to enlarge. |
Navigating the Facial Nerve
To better understand the various manifestations of FNP, it’s important to recall the anatomy of the facial nerve (Figure 1). The facial nerve nuclei are located in the pons: one motor nucleus (motor nucleus of the facial nerve), one parasympathetic nucleus (solitary nucleus) and one sensory nucleus (superior salivatory nucleus).5
The fibers from these nuclei can be afferent or efferent, visceral or somatic, and general or special, as described here:
- General somatic efferent (GSE) fibers provide motor supply to the facial muscles;
- General visceral efferent (GVE) fibers provide parasympathetic innervation to the salivary glands and lacrimal gland;
- Special visceral afferent (SVA) fibers carry taste sensation from the anterior two-thirds of the tongue; and
- General somatic afferent (GSA) fibers carry cutaneous sensations from the small area of skin over the mastoid process and the post-auricular area.
The axons exit the facial nerve nuclei and wrap around the abducens nucleus, pass through the 4th ventricle, and then exit the brainstem at the pontomedullary junction, next to CN VIII. The axons then pass through the posterior cranial fossa at the cerebellopontine angle, and then enter the internal acoustic meatus in the temporal bone. The axons then enter the Z-shaped bony facial canal and synapses at the geniculate ganglion. The greater superficial petrosal nerve arises from the geniculate ganglion, synapses in the pterygopalatine ganglion and provides parasympathetic innervation to the lacrimal gland (GVE). The facial nerve continues through the facial canal and gives off additional branches: the nerve to the stapedius muscle and the chorda tympani, which carries taste sensation (SVA) and parasympathetic innervation to the salivary glands (GVE). The facial nerve then exits the skull through the stylomastoid foramen. The posterior auricular nerve and the digastric nerve branch off. Finally, the facial nerve then enters the parotid gland, where it then divides into five branches (temporal, zygomatic, buccal, mandibular, cervical) which provide motor innervation to the facial muscles.5,6
Localize the Problem
A supranuclear lesion involves the motor cortex, subcortex or the corticobulbar tracts. Recall that the facial nerve nuclei have bilateral innervation from upper motor neurons to the upper face but only contralateral input into the lower face. Thus, a supranuclear lesion will result in FNP of the lower face only.
A nuclear CN VII lesion typically presents as FNP with ipsilateral CN VI palsy due to their proximity. Millard-Gubler syndrome is an eponym for a ventral pontine lesion involving CN VI, CN VII, and the corticospinal tracts, resulting in ipsilateral CN VI and VII palsy and contralateral hemiparesis.
A cerebellopontine angle lesion presents as a FNP with CN V, CN VI and CN VIII involvement. CN IX and X are in the inferior portion of the cerebellopontine angle. Differentiating symptoms of this localized FNP include the loss of the corneal reflex (CN V) with hearing loss and vertigo (CN VIII).2
Etiology
Six broad categories of FNP etiology are idiopathic, traumatic/iatrogenic, infectious, neoplastic, congenital and miscellaneous. The history and presentation play a critical role in identifying the cause of the paresis. Let’s dive further:
• Idiopathic. Bell’s palsy is the most common cause of FNP and is a diagnosis of exclusion. Characteristic findings include an abrupt onset of unilateral facial paresis that progresses within one to three days, history of a recent viral illness and involvement of both the upper and lower parts of the face. True Bell’s palsy involves all five branches of the facial nerve, causing paresis from hairline to the clavicle. Additional symptoms include ipsilateral earache, numbness of the face, tongue and ear; and, more rarely, hyperacusis, tinnitus, altered taste and reduced lacrimation. In rare occurrences, a neoplastic etiology can masquerade as Bell’s palsy, but the defining feature of Bell’s palsy is spontaneous improvement within three to four weeks, with complete resolution around six months from onset.7
Bell’s palsy is thought to be due to inflammation of the facial nerve from recent viral illness. This hypothesis is supported by the increased incidence of Bell’s palsy in pregnant women, about 45 per 100,000 compared to 17 per 100,000 in the non-pregnant.4 This could be attributed to pregnancy being a pro-inflammatory state. Herpes simplex virus has also been thought to cause Bell’s palsy, but this hasn’t been definitely proven.7
• Trauma/Iatrogenic. The second leading cause of FNP is trauma.4,7 Craniofacial trauma, most commonly from motor vehicle accidents, can cause blunt or penetrating trauma to the nerve. Notably, immediate onset FNP from a temporal bone fracture is associated with poor recovery, whereas incomplete FNP can have near complete recovery.
Iatrogenic injury to the facial nerve can occur from forceps delivery of neonates, orthognathic surgery, head and neck surgery for parotid tumors, acoustic neuromas, facial nerve schwannoma and other regional tumors.7
• Infectious. Ramsey-Hunt Syndrome (RHS), also known as geniculate ganglionitis, is caused by the herpes zoster virus reactivating in the geniculate ganglion of CN VII. It classically presents with a triad of ipsilateral FNP, otalgia and vesicles in the auditory canal. There’s a predominance of otologic symptoms of tinnitus, hypoacusis, vertigo and nystagmus, due to the proximity to CN VIII. While auditory canal vesicles are strongly associated with RHS, approximately 2 to 35 percent of cases of FNP without vesicles are due to herpes zoster infection as well. Therefore, it’s important to note that vesicles can develop at any point before, during or after the onset of FNP.2 Patients with RHS often have more severe disease and incomplete recovery.4,7
Other infectious etiologies include Lyme disease and otitis media. Less common infectious causes include tuberculous chronic otitis media, HIV, polio, mumps, leprosy, infectious mononucleosis, syphilis and botulism. FNP can be the first presenting sign of AIDS, and a detailed history is imperative to including this in the differential.2 Additionally, if there is presence of FNP with an ear infection, a cholesteatoma should be considered as the primary source causing infection and nerve compression.7
• Neoplastic. There should be high clinical suspicion for a tumor-associated FNP if there’s a slow progression of symptoms, no improvement in function after six months, multiple cranial nerve involvement, and/or recurrent ipsilateral palsy. The most common tumor affecting facial nerve function is an acoustic neuroma, and the most common tumor of the facial nerve is a facial schwannoma.7 Paralysis of the facial nerve from a facial schwannoma can be preceded by facial spasms.
Malignant parotid neoplasms can cause FNP from direct facial nerve invasion or after tumor extirpation. Nasopharyngeal carcinoma can involve the pterygopalatine ganglion which can present with reduced lacrimation due to involvement of the greater superficial petrosal nerve.7
• Congenital. Congenital FNP should be considered in newborns without iatrogenic injury. Syndromes associated with congenital FNP include:
—Moebius syndrome – Congenital CN VI and VII palsy resulting in horizontal gaze palsy and masked facies. It’s thought to be due to brainstem insult during development.8
—Goldenhar syndrome – Abnormal development of the 1st and 2nd branchial arches which results in incomplete development of the ear with resultant facial nerve hypofunction, along with mandibular hypoplasia causing facial asymmetry, ear anomalies, eye anomalies, and vertebral malformation. It’s considered a more severe form of oculo-auricular-vertebral spectrum.9,10
—DiGeorge Syndrome – A deletion in Chromosome 22q11.2 causing abnormal development of the 3rd and 4th branchial arches. Interestingly, this can also lead to maldevelopment of the 1st and 2nd branchial arches. Useful mnemonic: CATCH-22, which stands for Cono-truncal Cardiac abnormalities, Abnormal facies, Thymic hypoplasia, Cleft palate/cellular immunodeficiency, Hypoparathyroidism with hypocalcemia.11
—CHARGE Syndrome – The predominant findings in CHARGE syndrome are: Coloboma, Heart defect, Atresia choanae, Retarded growth and development, Genital hypoplasia and Ear anomalies/deafness. It has a strong association with at least one anomalous cranial nerve (CN IX/X > CN VII > CN VIII).12
Miscellaneous Causes
Etiologies that don’t fall in the other categories include:
• Systemic and metabolic disorders. Such disorders associated with FNP are diabetes mellitus, hypertension, amyloidosis, and sarcoidosis.3
• Neurological disorders. Conditions that have been reported to cause FNP are Guillan-Barre syndrome, myasthenia gravis, multiple sclerosis and cerebrovascular accidents.3
• Melkersson-Rosenthal syndrome. This is a rare disorder that presents with a classic triad of orofacial edema, fissured tongue and recurrent, alternating FNP. Lip biopsy shows Langerhans giant cells and non-caseating granulomas.3
• Autoimmune causes. These include rheumatoid arthritis, Sjogren’s syndrome, lupus and sarcoidosis.3
Diagnosis
Here are the salient points to keep in mind when evaluating a patient for possible FNP:
• History. Accurate diagnosis relies on a detailed history and physical examination. Timing of presentation and progression are critical to differentiating between a neoplastic versus a non-neoplastic process. Associated symptoms of dizziness, dysphagia, diplopia, reduced lacrimation, altered taste sensation, hyperacusis or hearing loss all assist in localizing the affected part of the facial nerve.7
• Clinical Exam. A targeted ophthalmic, neurologic, and otologic examination helps to identify the extent of facial nerve paralysis and narrow the differential. Here are the highlights of the ophthalmologic examination:
— baseline visual acuity, pupillary exam and extraocular movement;
— Schirmer’s test: less than 10 mm indicates insufficiency and impairment at or above the great superficial petrosal nerve;
— corneal reflex: impaired ipsilateral reflex is an early sign of cerebellopontine angle syndrome;13
— examine the forehead and brow, checking frontalis strength and brow position. If the frontalis muscle is spared in FNP, suspect a central palsy. If there’s complete paralysis of the upper and lower face, it’s likely a peripheral palsy;
— examine the lids, specifically looking at blink rate, orbicularis strength, upper lid retraction, lower lid ectropion and lagophthalmos;
— check the strength of the Bell’s phenomenon; a strong Bell’s phenomenon will be protective to the cornea;
— at the slit lamp, assess the cornea for dryness, thinning, epithelial defects, ulceration; and
— on the fundus exam, look for diabetic or hypertensive changes in the retina and disc edema.
Your focused neurologic examination should consist of the following:
— Examine neighboring cranial nerves and compare them to the contralateral side, and at rest.
– Test facial sensation in CN V1-V3 dermatomes.
– Assess CN VI function by examining extraocular movement.
– FNP with ipsilateral CN VI palsy is suggestive of a pontine lesion.
– Look for nystagmus, which could suggest CN VIII involvement.
– Test hearing grossly (CN VII and VIII) by rubbing your fingers by the patient’s ears.
– Hyperacusis suggests CN VII dysfunction by loss of stapedius muscle function.
– Hearing loss suggests CN VIII involvement.
— Assess for development of synkinesis: aberrant regeneration after facial nerve injury resulting in simultaneous involuntary facial contractions with voluntary facial movement.
Focused otologic examination consists of:
— external examination of the face for abnormal prominences such as parotid or pre- or post-auricular inflammation;
— external ear examination to assess for vesicles; and
— palpation of the mastoid process (tenderness suggests a middle ear infection).2
Click to enlarge. |
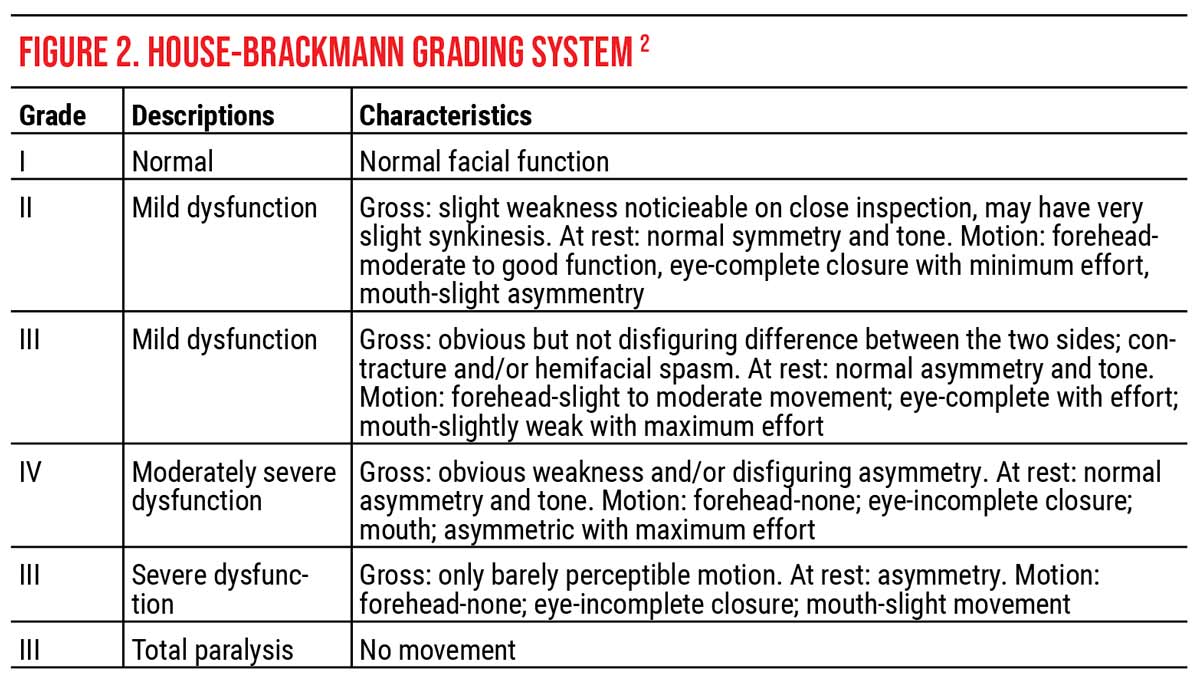
Grading Systems
Grading FNP is an important way to communicate among multi-disciplinary teams and track functionality. The gold standard is the modified House-Brackmann grading system (HBGS) adopted by the American Academy of Otolaryngology-Head and Neck Surgery in 1984. This scale takes measurements at the affected side eyebrow, and corner of the mouth, with each 0.25-cm movement corresponding to 1 point, with a maximum score of 4. The two values are added and this scale of 0 to 8 is converted to the I to VI grading system (Figure 2). The HBGS assesses gross facial motion based on a scale of I (normal facial nerve function) to VI (complete absence of facial nerve function).14
Other well-known grading schemas are the Nottingham system, Sydney system and the Sunnybrook Scale. One study showed good consistency between Sydney and Sunnybrook, but the assessment of synkinesis was less reliable. The reliability was high for House-Brackmann, but there was wide variation in trained practitioners.15
Work-up
Indications for work-up include: 1) inability to recall date of onset; 2) multiple cranial nerve involvement; 3) persistence of paralysis more than six months; or 4) progression or recurrence of “Bell’s palsy.” If a patient has an acute onset of complete unilateral facial paralysis developing over the course of one to three days, this is consistent with Bell’s palsy, and no additional testing or imaging is necessary.
• Laboratory testing. Lyme titers (in endemic areas), syphilis serologies and EBV testing may be considered for infectious etiologies.4 An autoimmune work-up including ACE, lysozyme, chest X-ray, ANA, SSA, SSB and RF may be considered.4 Consider biopsy of tissue adjacent to the facial nerve if paresis persists for more than six or seven months, or if there is progression or recurrence.2
• Imaging. For trauma associated FNP, the best modality to evaluate bone is computed tomography. For all other diagnoses, imaging should start with an MRI of the brain and brainstem with attention to the facial nerve path.
In conclusion, the facial nerve has an intricate path, but an understanding of the anatomy can help localize the lesion. While Bell’s palsy is the most common cause of FNP, other more sinister causes must be ruled out. The ophthalmologist is a key player in the work-up and management, as these patients often present to us first. Stay tuned for the second part of our series on FNP where we will discuss management.
Dr. Lao is an assistant professor of ophthalmology and neurology at the University of Tennessee Health Science Center/Hamilton Eye Institute in Nashville.
Dr. Mukit is a resident physician, PGY-3, at the Department of Ophthalmology at the University of Tennessee Health Science Center/Hamilton Eye Institute.
Dr. Noguera is an oculofacial plastics and reconstructive surgery fellow at UT Health Science Center/HEI.
Riley Bastian is a third-year medical student at Northeast Ohio Medical University.
1. Sajadi MM, Sajadi MR, Tabatabaie SM. The history of facial palsy and spasm: Hippocrates to Razi. Neurology 2011;77:2:174-178.
2. Portelinha J, Passarinho MP, Costa JM. Neuro-ophthalmological approach to facial nerve palsy. Saudi J Ophthalmol 2015;29:1:39-47.
3. MacIntosh PW, Fay AM. Update on the ophthalmic management of facial paralysis. Surv Ophthalmol 2019;64:1:79-89.
4. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: A review. Surv Ophthalmol 2007;52:2:121-144.
5. Seneviratne SO, Patel BC. Facial Nerve Anatomy and Clinical Applications. [Updated 2022 Jul 25]. In: StatPearls [online publication]. Treasure Island, Fla.: StatPearls Publishing, 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554569/
6. Dulak D, Naqvi IA. Neuroanatomy, Cranial Nerve 7 (Facial) [Updated 2022 Jul 25]. In: StatPearls [online publication]. Treasure Island, Fla.: StatPearls Publishing, 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526119/
7. Alsuhaibani AH. Facial nerve palsy: Providing eye comfort and cosmesis. Middle East Afr J Ophthalmol 2010;17:2:142-147.
8. Singham J, Manktelow R, Zuker RM. Möbius syndrome. Semin Plast Surg 2004;18:1:39-46.
9. González-Rodríguez B, González-Rodríguez M. Goldenhar syndrome. Am J Ophthalmol 2021;232:e3.
10. Logan G, Sheikh O, Pabla R, Ayliffe P. Goldenhar's syndrome: A case series with literature review and update. Journal of Oral and Maxillofacial Surgery 2013;71:9:e47.
11. Puñal EJ, Siebert MF, Angueira FB, Castro-Gago M. Three new patients with congenital unilateral facial nerve palsy due to chromosome 22q11 deletion. Journal of Child Neurology 2001;16:6:450-452.
12. Blake KD, Hartshorne TS, Lawand C, Dailor AN, Thelin JW. Cranial nerve manifestations in CHARGE syndrome. Am J Med Genet A 2008;146A:5:585-592.
13. Mavrikakis I. Facial nerve palsy: Anatomy, etiology, evaluation, and management. Orbit 2008;27:6:466-474.
14. Kang TS, Vrabec JT , Giddings N, Terris, D. J. Facial nerve grading systems (1985–2002): Beyond the House-Brackmann Scale. Otology & Neurotology 2002;23:5:767.
15. Coulson SE, Croxson GR, Adams RD, O'Dwyer NJ. Reliability of the "Sydney," "Sunnybrook," and "House Brackmann" facial grading systems to assess voluntary movement and synkinesis after facial nerve paralysis. Otolaryngol Head Neck Surg 2005;132:4:543-549.