The diagnosis of ocular toxocariasis can be challenging, both because the condition is relatively uncommon and because its presentation typically varies from patient to patient. In a recent study of the prevalence, clinical features and causes of vision loss in patients with ocular toxocariasis done at the Francis I. Proctor Foundation, University of California, San Francisco, the overall prevalence of Toxocara infection of the eye was relatively low at 1 percent of a general uveitis population.1 Nevertheless, ocular toxocariasis remains an important cause of uveitis, especially in children and young adults.
Human infection by the nematodes Toxocara canis or Toxocara cati can cause both systemic and ocular toxocariasis. The definitive hosts for these species of nematodes are dogs and cats, respectively. Humans acquire the disease by ingestion of embryonated eggs, usually from contaminated raw vegetables or infected raw meat (chicken, rabbit and lamb),2 contaminated water sources or via geophagia. Most ocular damage results from the inflammatory response that occurs following death of the larva. For reasons that are not well-understood, ocular and systemic toxocariasis are generally diagnosed in patients of different ages. Specifically, it has been observed that systemic toxocariasis, also called visceral larva migrans, tends to affect children younger than 3 years of age, possibly due to an increased rate of pica among this group,3 whereas ocular toxocariasis typically presents in older children and young adults.
Clinical Presentation
Ocular toxoxcariasis is unilateral in 90 percent of patients.1,4,5 The condition has traditionally been taught to affect primarily infants and young children, but recent surveys have reported Toxocara infections in a number of teenagers and young adults, as well. Whether this shift in demographics has been due to an increased understanding of the epidemiology of ocular toxocariasis, a change in referral patterns, or a true change in the populations at risk is unknown.1,5 Contact with domestic dogs and cats, especially puppies and kittens, which are more likely to carry the infection, is considered an important risk factor for children. Common symptoms that bring patients to the clinic include blurred vision and floaters. Pain and photophobia may also be present but are typically mild.6 In young patients, the eye infection may not be noticed until they fail a school vision screening test, or develop strabismus or leukocoria. Figure 1. Two separate patients (A and B) with healed posterior pole granulomas due to ocular toxocariasis. Note the prominent retinal folds and epiretinal membrane formation.
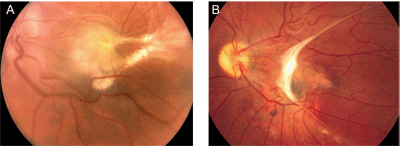
Ocular toxocariasis presents as a posterior pole granuloma (See Figure 1) in 25 percent to 50 percent of patients.1,4,5,6 The reason for such an apparent predilection for the posterior pole is unclear, although it has been suggested that hematogenously spread nematodes may be more likely to lodge in small, perifoveal end-arteries. Conversely, it may be simply that macular lesions are most likely to be symptomatic and, hence, prompt patients to seek medical attention. Acutely, Toxocara retinochoroiditis appears as a hazy, ill-defined white lesion with an overlying vitritis. As the inflammation resolves the lesion is seen as a distinct, well-demarcated, elevated white mass ranging from one-half to four disc diameters in size. A retinal pigment epithelium disturbance often surrounds the lesion and retinal folds extending from the lesion are quite common. Close examination may reveal a dark grey center, which has been proposed to be remnants of the larva,6,7 as well as retinal blood vessels entering the granuloma. Toxocara granulomas can also develop on the optic disc, in which case vision loss may be profound; an afferent pupillary defect may be present, and fundus examination may reveal a peripapillary detachment and/or retinal exudates in the pattern of a macular star (neuroretinitis).
About 50 percent of all eyes with toxocariasis present as a peripheral granuloma (See Figure 2).1,4,5,6 In the acute form, the lesion appears as a hazy, white mass in the peripheral fundus that may mimic the sort of snowbank seen in patients with pars planitis. Over time, consolidation and contraction of the lesion produces a peripheral, elevated mass, often with posteriorly extending retinal folds (See Figure 3). Retinal exudates may be extensive in some instances.
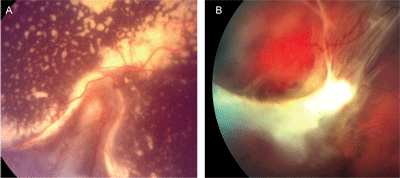
Figure 2. Two separate patients with peripheral granulomas due to ocular toxocariasis. Note the prominent, posteriorly extending retinal folds and, in case A, extensive exudate.
Less than 25 percent of eyes with toxocariasis present with dense vitreous inflammation mimicking endophthalmitis (See Figure 3).1,4,6 Unlike many other forms of uveitis, the signs of Toxocara endophthalmitis are often more pronounced than the symptoms. In fact, as noted, pain and photophobia are usually minimal or absent. Slit-lamp examination typically reveals a mild to moderate spillover anterior chamber reaction with scattered small keratic precipitates on the corneal endothelium. Posterior synechiae may develop occasionally, but are uncommon. Hypopyon formation, although reported, is rare.8 Fundus visualization through the vitreous inflammation is usually difficult. Severe cases may develop an exudative retinal detachment. As the inflammation subsides, vitreous membranes may organize into a retrolental mass, which in severe cases can produce ciliary body detachment, hypotony and phthisis bulbi.
The primary causes of vision loss in patients with ocular toxocariasis depend to a large extent on the location and severity of the inflammation. Vision is typically decreased in patients with Toxocara endophthalmitis due to inflammatory media opacities, cystoid macular edema and/or cataract formation. Posterior pole granulomas, in contrast, usually cause vision loss by direct involvement of the macula or optic disc, by secondary formation of retinal folds or epiretinal membranes, or, rarely, by the development of choroidal neovascularization. Decreased vision in patients with peripheral ocular toxocariasis typically results from macular involvement by posteriorly extending falciform folds or exudate. In addition, amblyopia may develop in young patients with media opacities and/or macular involvement. Figure 3. Toxocara endophthalmitis. Extensive vitritis obscures fundus details such that the optic disc (arrow) is barely visible.

Diagnosis
While history, review of systems and a complete, dilated eye examination are most important, several ancillary tests can also be helpful in diagnosing ocular toxocariasis. Serum eosinophilia is suggestive of infection, but is usually only present in the setting of an active systemic infection. Eosinophils detected in aqueous or vitreous fluid are more direct indicators of ocular infection, but such fluids are rarely available for testing. Assays to detect anti-Toxocara antibodies in the serum are employed most routinely and can be valuable to support the clinical diagnosis, but are notoriously insensitive. Improved sensitivity can be achieved by using an ELISA analysis of intraocular fluids,9,10 but such testing is only available at a few isolated research facilities. For all intents and purposes, therefore, ocular toxocariasis is often a clinical diagnosis.
Imaging modalities can provide additional information in many patients. Optical coherence tomography of posterior pole granulomas usually demonstrates a highly reflective mass located above the RPE11 and can also show factors potentially contributing to vision loss, such as the presence of intraretinal or subretinal fluid. B-scan ultrasonography is particularly helpful when vitritis or other opacities preclude a view of the fundus.
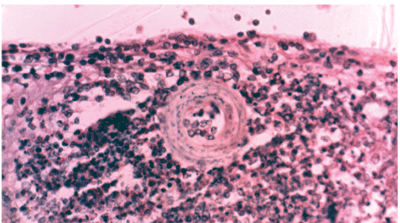
Rarely the diagnosis of ocular toxocariasis is made histopathologically following identification of a dead larva in the center of an eosinophilic granuloma (See Figure 4).
Treatment
The decision to start therapy depends on the stage of infection. Quiescent clinical presentations with no active inflammation require no anti-inflammatory or anti-helminthic treatment. For those rare patients who present with eye involvement in the setting of visceral larva migrans, systemic treatment with one of the benzimidazole derivatives (albendazole, thiabendazole and mebendazole) in conjunction with local and/or systemic corticosteroids as indicated is most effective.12,13 While no comparative trials are available, albendazole's better tolerance and general availability in most countries make it the anti-helminthic drug of choice in most settings.
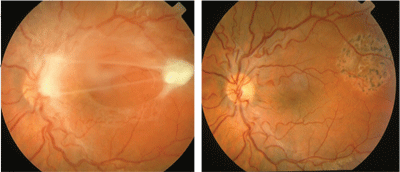
Corticosteroids can be administered topically, periocularly, intraocularly or systemically, depending on the location and severity of inflammation. A cycloplegic/mydriatic is often indicated when anterior chamber inflammation is present to minimize the risk of posterior synechiae formation and should be selected and dosed based on the severity of the inflammation. Pars plana vitrectomy should be considered in selected patients to remove inflammatory tissues, relieve vitreomacular traction, and repair retinal detachment (See Figure 5).14 Attempts have also been made to surgically remove live larva from the subretinal space.15 Alternatively, a motile larva can be destroyed using laser photocoagulation.
Prophylaxis
Proper disposal of pet litter, deworming infected pets, complete cooking of meats, thorough rinsing of fruits and vegetables, and good hand-washing practices are important measures for preventing the human infection.16 Covering sandpits in common playgrounds and outdoor vegetable gardens is also helpful, since it helps to prevent contamination and the temperature rise caused by covering independently promotes destruction of existing eggs.17
Figure 4. An H&E stained section through the peripheral retina showing a dead Toxocara canis larva in the center of an eosinophilic granuloma. The section was cut from the original histopathology block first studied and reported in 1965.19
Although not currently available, molecular vaccines could someday prove useful in controlling dog and cat infestation. So far the Toxocara myosins have been identified as potential antigen candidates for such a vaccine.18
A high index of suspicion is required to diagnose ocular toxocariasis. Children and young adults are affected most commonly. The inflammation is usually unilateral with one of three presentations: a posterior pole granuloma, a peripheral granuloma or Toxocara endophthalmitis. Serum testing for anti-Toxocara antibodies can be helpful, but is notoriously insensitive and so negative results should be interpreted with caution. Imaging, particularly OCT and B-scan ultrasonography, is often useful. Most patients with ocular toxocariasis benefit from treatment with corticosteroids, but not anti-helminthic agents, since inflammation results from the body's response to the dead worm. An anti-helminthic agent should be considered, however, in the setting of an active systemic infection or if a live, motile larva is seen ophthalmoscopically. Pars plana vitrectomy is indicated in selected patients with long-standing vitreous debris or traction or rhegmatogenous retinal detachment.14
Figure 5. A posterior pole granuloma in a 10-year-old girl with a pet skunk and a two month history of decreased vision and floaters affecting her left eye (A). Vision at presentation was 20/100. A serum anti-Toxocara IgG was positive at a 1:16 dilution. A pars plana vitrectomy was performed to excise the granuloma and remove the epiretinal membranes (B). Final post-operative vision was 20/25.
Dr. Singh is a clinical fellow and Dr. Stewart is on faculty in the Department of Ophthalmology, UCSF School of Medicine. Contact them at the Department of Ophthalmology, UCSF School of Medicine, 10 Koret Way, K301, San Francisco, Calif. 94143-0730, Phone: (415) 476-0496, fax: (415) 476-0336, e-mail: stewartj@vision.ucsf.edu.
Dr. Cunningham is director of the Uveitis Service, California Pacific Medical Center and a clinical professor of ophthalmology, Stanford University School of Medicine. Contact him at West Coast Retina Medical Group, Inc. 185 Berry St., Lobby 5, Suite 130, San Francisco, Calif. 94107-1739. Phone: (415) 972 4600, fax: (415) 975 0999, e-mail: emmett_cunningham@yahoo.com
1. Stewart JM, Cubillan LD, Cunningham ET Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina 2005;25:1005-13.
2. Nagakura K, Tachibana H, Kaneda Y, Kato Y. Toxocariasis possibly caused by ingesting raw chicken. J Infect Dis 1989;160:735-6.
3. Schantz PM, Weis PE, Pollard ZF, et al. Risk factors for toxocaral ocular larva migrans: A case control study. Am J Public Health 1980;70:1269.
4. Wilkinson CP, Welch RB. Intraocular toxocara. Am J Ophthalmol 1971;71:921-30.
5. Yokoi K, Goto H, Sakai J, Usui M. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm 2003;11:269-75.
6. Shields JA. Ocular toxocariasis. A review. Surv Ophthalmol 1984;28:361-81
7. Foster CS. Ocular Toxocariasis. In: Foster and Vitale. Diagnosis and treatment of uveitis. Philadelphia: W.B. Saunders, 2002:428-436.
8.Appelmans M, Michiels J, Roquet P. Uveitis with eosinophilic hypopyon caused by nematode larvae. Bull Soc Belge Ophtalmol 1965;140:505-12
9. Magnaval JF, Malard L, Morassin B, Fabre R. Immunodiagnosis of ocular toxocariasis using Western-blot for the detection of specific anti-Toxocara IgG and CAP for the measurement of specific anti-Toxocara IgE. J Helminthol 2002;76:335-9.
10.Benitez del Castillo JM, Herreros G, Guillen JL. Bilateral ocular Toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol 1995;119:514-6.
11. Higashide T, Akao N, Shirao E, Shirao Y. Optical coherence tomographic and angiographic findings of a case with subretinal toxocara granuloma. Am J Ophthalmol 2003;136:188-90.
12. Sturchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: A clinical trial. Ann Trop Med Parasitol 1989;83:473-8.
13. Sabrosa NA, de Souza EC. Nematode infections of the eye: Toxocariasis and diffuse unilateral subacute neuroretinitis. Curr Opin Ophthalmol 2001;12:450–454.
14. Amin HI, McDonald HR, Han DP, Jaffe GJ, et al. Vitrectomy update for macular traction in ocular toxocariasis. Retina 2000;20:80-5.
15. de Souza EC, Nakashima Y. Diffuse unilateral subacute neuroretinitis. Report of transvitreal surgical removal of a subretinal nematode. Ophthalmology 1995;102:1183-6.
16. Harvey JB, Roberts JM, Schantz PM. Survey of veterinarians' recommendations for treatment and control of intestinal parasites in dogs: Public health implications. J Am Vet Med Assoc 1991;199:702-7.
17. Uga S, Kataoka N. Measures to control Toxocara egg contamination in sandpits of public parks. Am J Trop Med Hyg 1995;52:21-4.
18. Obwaller A, Duchene M, Bruhn H, Steipe B, Tripp C, Kraft D, Wiedermann G, Auer H, Aspock H. Recombinant dissection of myosin heavy chain of Toxocara canis shows strong clustering of antigenic regions. Parasitol Res 2001;87:383-9.
19. Hogan MJ, Kimura SJ, Spencer WH. Visceral larva migrans and peripheral retinitis. JAMA 1965;194:1345-7.



