Presentation and Initial Work-Up
A 36-year-old male was referred to Wills Eye Hospital for evaluation of poor vision after an injury to his right eye. He had undergone LASIK in India in both eyes 10 years prior, with subsequent trauma to the right eye from a 3x5 index card one year afterwards. He was seen and evaluated by multiple ophthalmologists, initially undergoing a trial of rigid gas permeable lenses which were stopped due to discomfort. He reported that vision in the right eye had been poor since the injury; vision in the left eye had been stable. At the time of evaluation, he denied pain, redness or discharge.
Medical History
 |
|
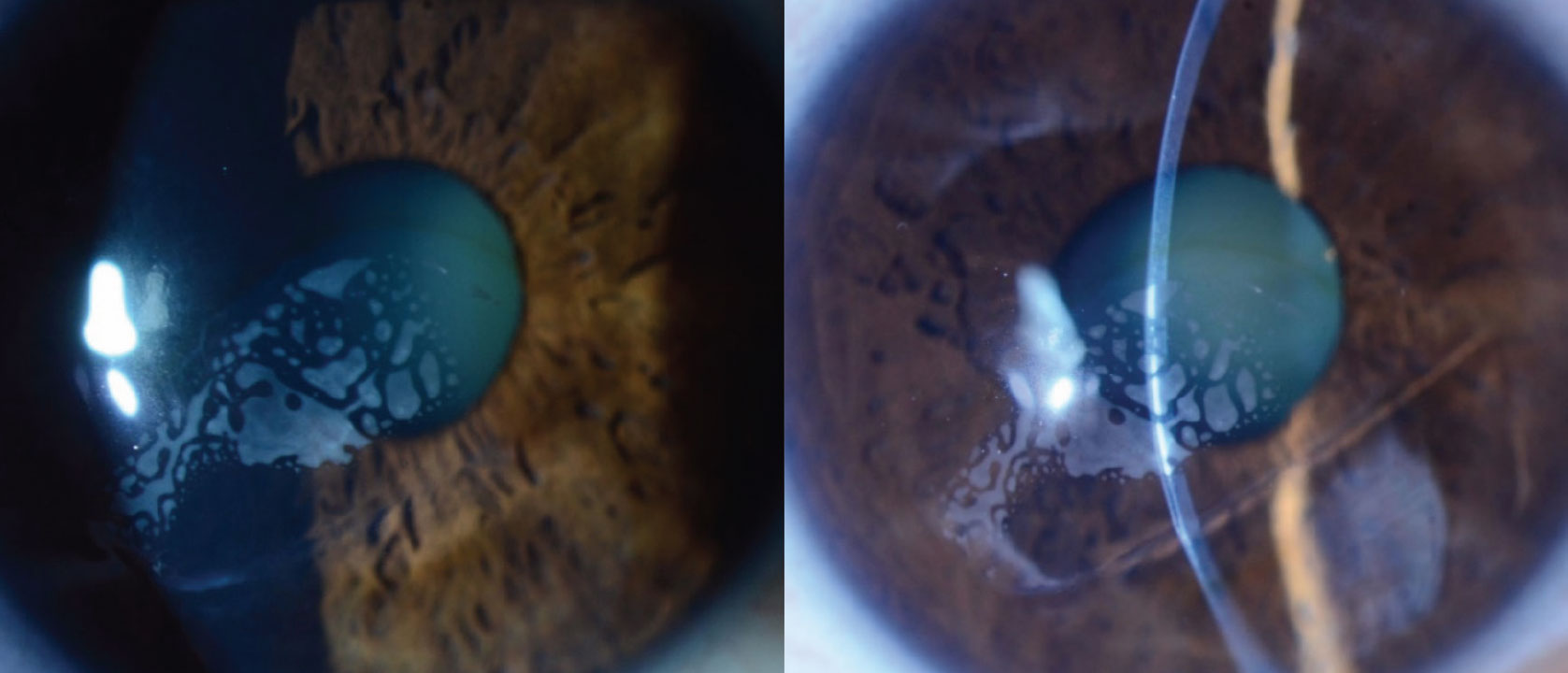
Figure 1. Slit lamp examination showing a large area of gray-white opacities in the LASIK flap interface, an iron line, missing flap inferiorly and irregular corneal curvature. Click image to enlarge. |
Past medical history was noncontributory and he had no other surgical history. Family history was negative for chronic conditions. He denied alcohol, tobacco and illicit drug use.
Exam
Uncorrected visual acuity was 20/400 in the right eye, without improvement with refraction but with pinhole improved to 20/150, and 20/20 in the left eye. Pupillary examination was normal and confrontational visual fields and extraocular movements were full bilaterally. Intraocular pressure was within normal limits bilaterally.
Anterior slit lamp examination of the right eye showed a LASIK flap with significant gray-white opacities in the flap interface measuring 5.7 x 2.3 mm, an iron line, an irregular corneal surface and a missing flap inferiorly (Figure 1). Anterior slit lamp examination of the left eye was unremarkable, and showed a LASIK flap without abnormalities.
What is your diagnosis? What further workup would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
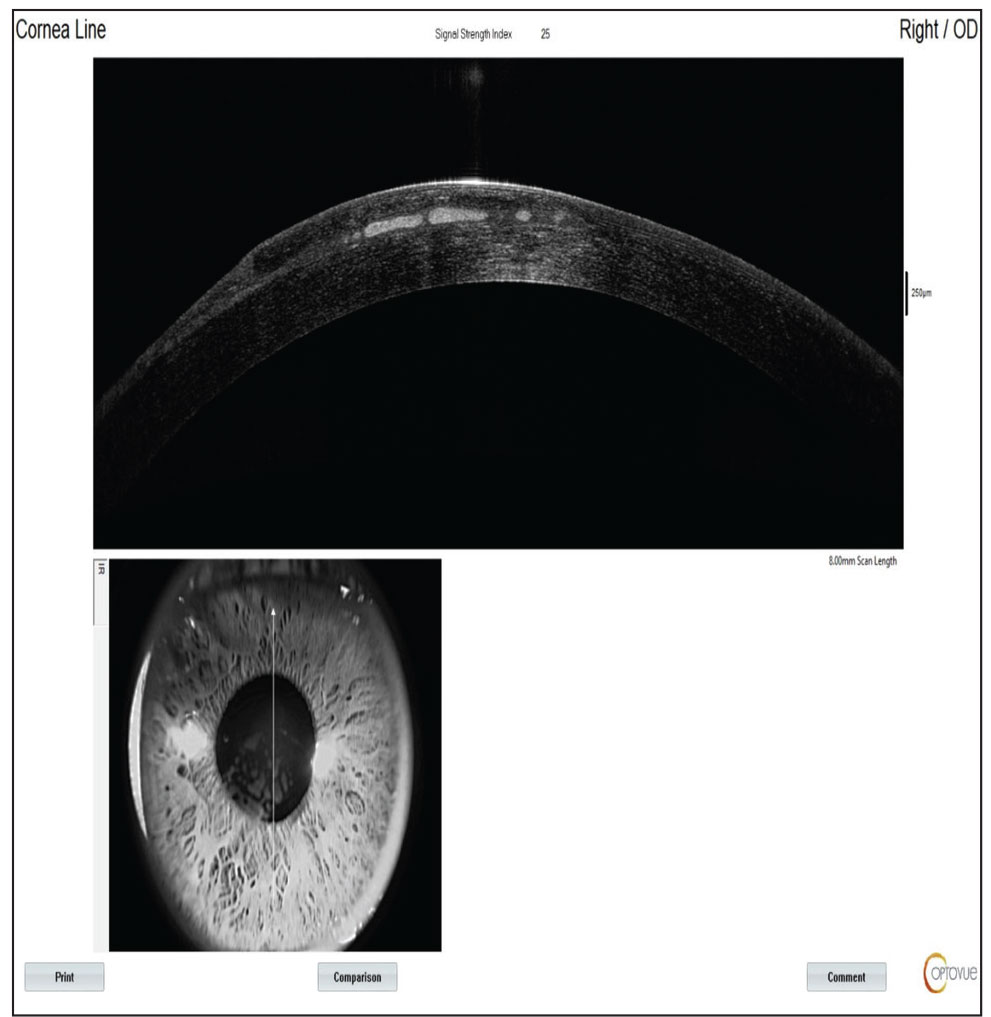 |
| Figure 2. AS-OCT demonstrating an irregular LASIK flap and anomalous tissue within the flap interface. Click image to enlarge. |
Anterior segment optical coherence tomography revealed an irregular corneal flap and anomalous tissue within the flap interface (Figure 2). Corneal topography and tomography maps showed significant irregular astigmatism and an area of inferior steepening at the inferior paracentral zone, with inferotemporal thinning (Figure 3).
Based on clinical and multimodal imaging features, the patient was found to have significant epithelial ingrowth with a partially missing LASIK flap in the right eye. He was presented with options to manage clinically significant epithelial ingrowth, including observation, rigid gas permeable contact lens, scleral lens, laser treatment, attempting to lift the flap and scrape the epithelial ingrowth, amputate the flap and treat with mitomycin C, and partial- or full-thickness corneal transplant. He elected to avoid surgery and observe for now, with frequent evaluation for progression, approximately every six months.
Discussion
Post-LASIK epithelial ingrowth is due to the growth of surface epithelial cells under the LASIK flap, resulting from poor flap adhesion and subsequent invasion of the epithelial cells. When severe enough, patients commonly experience blurred vision, glare or halos, visual distortion, pain and/or foreign body sensation. Diminished visual acuity results from these cells causing irregular astigmatism, frank opacity in the visual axis, and/or decreased nutrition delivery to healthy keratocytes, resulting in stromal melt.
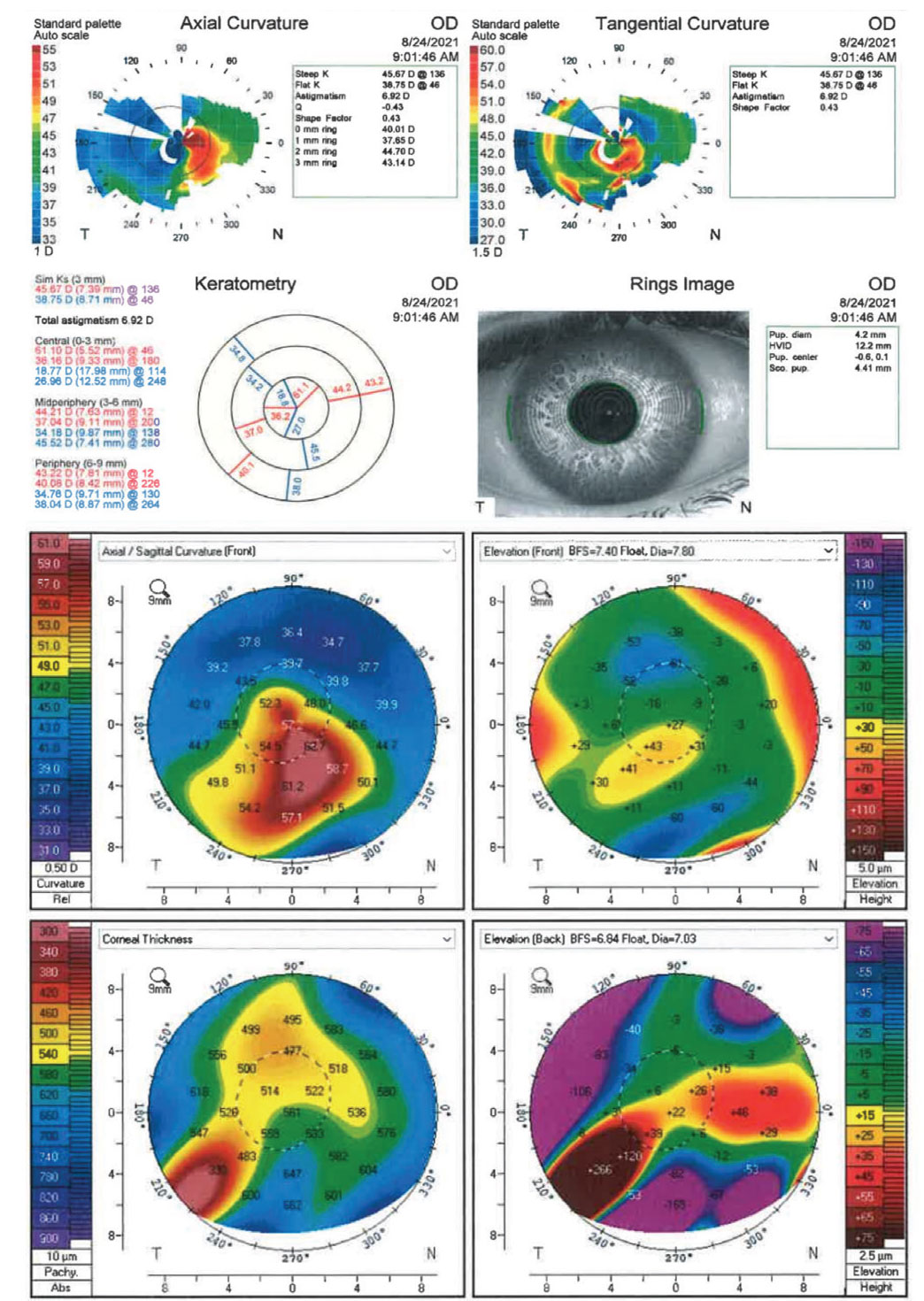 |
| Figure 3. Topography and tomography maps demonstrating significant irregular astigmatism. Click image to enlarge. |
Clinically significant epithelial ingrowth is a relatively uncommon complication after primary LASIK; one study found an incidence of 0 percent in 3,866 cases.1 The most common etiologic factor is LASIK retreatment, with an incidence ranging from 2 percent to 20 percent.1–3 In particular, clinically significant ingrowth seems to occur more frequently when LASIK retreatment is performed three or more years after primary LASIK.1 In the post-LASIK setting, decreased visual acuity may also be secondary to other interface complications such as infectious keratitis and diffuse lamellar keratitis; thus, epithelial ingrowth must be distinguished from these conditions. Epithelial ingrowth is generally easily distinguishable on slit lamp examination due to the appearance of nests of cells at the flap interface.
Potential risk factors for epithelial ingrowth include: the method of flap creation; flap thickness; a postoperative epithelial defect; diffuse lamellar keratitis; epithelial basement membrane dystrophy; a history of recurrent erosions; chronic eye rubbing; Meibomian gland disease; and especially flap trauma.4,5,6,7 Femtosecond-laser-assisted flaps have been shown to result in less epithelial ingrowth than mechanical microkeratome flaps, which may be related to the anatomy of the flap edge.4,8
Severity of epithelial ingrowth is highly variable; it can be asymptomatic, self-limited or clinically significant and in need of treatment for irregular astigmatism and prevention of flap melt.9 One review of 55 eyes with post-LASIK epithelial ingrowth showed that mild epithelial ingrowth may be safely observed, while significant epithelial ingrowth that reaches several millimeters towards the pupillary margin can respond well to removal.10 Treatment may be categorized as non-surgical, laser or surgical. Laser treatment includes yttrium-aluminum-garnet (YAG) laser, which, when applied to the region of ingrowth, creates gas bubbles that destroy the epithelial ingrowth cells.7
Surgical options include flap lift and mechanical scraping, flap removal and even partial- or full-thickness corneal transplantation.11 Flap lift and scraping is commonly used, and involves mechanical debridement of the posterior flap and stromal bed. However, recurrence after flap lift and scraping is relatively high, at 23 to 36 percent.2,5 Application of alcohol after flap lift and scraping has been employed to destroy residual epithelial cells, but this can also be damaging to the cornea. Excimer laser phototherapeutic keratectomy of the interface has also been used in an attempt to eliminate residual epithelial cells, but it can result in irregular astigmatism. Mitomycin C may also be used to reduce corneal haze. Suturing the flap edge after mechanical scraping of the interface is often successful in treating epithelial ingrowth.10 Additional use of hydrogel sealant or fibrin glue as an adjunct after thorough debridement may also be helpful in preventing recurrence.12–16
A complication to be aware of with epithelial ingrowth is the possibility of corneal flap melt, which can occur in as little as two weeks’ time. It’s thought to be secondary to lack of nutrients reaching the flap and/or collagenase that’s released from hypoxic epithelial cells underneath the flap. In this patient, the missing portion of inferior flap may have been a result of flap melt from a prolonged period of epithelial ingrowth.
In summary, we describe a patient with clinical and imaging findings of severe epithelial ingrowth with a portion of missing LASIK flap years after eye trauma. We summarize the disease pathogenesis, risk factors and multitude of management options for this condition. While there is no one-size-fits-all treatment for this condition, depending on the severity and location of epithelial ingrowth, there are promising options for managing it.
1. Caster AI, Friess DW, Schwendeman FJ. Incidence of epithelial ingrowth in primary and retreatment laser in situ keratomileusis. J Cataract Refract Surg 2010;36:1:97-101.
2. Henry CR, Canto AP, Galor A, Vaddavalli PK, Culbertson WW, Yoo SH. Epithelial ingrowth after LASIK: Clinical characteristics, risk factors, and visual outcomes in patients requiring flap lift. J Refract Surg 2012;28:7:488-92.
3. Ting DSJ, Srinivasan S, Danjoux JP. Epithelial ingrowth following laser in situ keratomileusis (LASIK): Prevalence, risk factors, management and visual outcomes. BMJ Open Ophthalmol 2018 29;3:1:e000133. doi: 10.1136/bmjophth-2017-000133. PMID: 29657982; PMCID: PMC5895975.
4. Letko E, Price MO, Price FW Jr. Influence of original flap creation method on incidence of epithelial ingrowth after LASIK retreatment. J Refract Surg 2009;25:11:1039-41.
5. Wang MY, Maloney RK. Epithelial ingrowth after laser in situ keratomileusis. Am J Ophthalmol 2000;129:6:746-51.
6. Asano-Kato N, Toda I, Hori-Komai Y, et al. . Epithelial ingrowth after laser in situ keratomileusis: Clinical features and possible mechanisms. Am J Ophthalmol 2002;134:801–7.
7. Ayala MJ, Alió JL, Mulet ME, De La Hoz F. Treatment of laser in situ keratomileusis interface epithelial ingrowth with neodymium:yytrium-aluminum-garnet laser. Am J Ophthalmol 2008;145:4:630-634.
8. Kamburoğlu G, Ertan A. Epithelial ingrowth after femtosecond laser-assisted in situ keratomileusis. Cornea 2008;27:10:1122-5.
9. Randleman JB, Shah RD. LASIK interface complications: Etiology, management, and outcomes. J Refract Surg 2012;28:8:575-86.
10. Rapuano CJ. Management of epithelial ingrowth after laser in situ keratomileusis on a tertiary care cornea service. Cornea 2010;29:3:307-13.
11. Kymionis G, Ide T, Yoo S. Flap amputation with phototherapeutic keratectomy (PTK) and adjuvant mitomycin C for severe post-LASIK epithelial ingrowth. Eur J Ophthalmol 2009;19:2:301-3.
12. Yeh DL, Bushley DM, Kim T. Treatment of traumatic LASIK flap dislocation and epithelial ingrowth with fibrin glue. Am J Ophthalmol 2006;141:5:960-2.
13. Anderson NJ, Hardten DR. Fibrin glue for the prevention of epithelial ingrowth after laser in situ keratomileusis. J Cataract Refract Surg 2003;29:7:1425-9.
14. Hirabayashi KE, Manche EE. Hydrogel sealant to prevent recurrent epithelial ingrowth in the setting of a LASIK flap buttonhole. Am J Ophthalmol Case Rep 2019;17:15:100518.
15. Yesilirmak N, Diakonis VF, Battle JF, Yoo SH. Application of a hydrogel ocular sealant to avoid recurrence of epithelial ingrowth after LASIK enhancement. J Refract Surg 2015;31:4:275-7.
16. Ramsook SS, Hersh PS. Use of a hydrogel sealant in epithelial ingrowth removal after laser in situ keratomileusis. J Cataract Refract Surg 2015;41:12:2768-71.



