Presentation
A 74-year-old African-American female presented to the Wills Eye Emergency Room with a five-day history of severe vision loss in the right eye. The patient noted vision loss upon awakening with progressive worsening. The left eye was unaffected. Review of systems was positive for recent-onset shoulder and hip pain, as well as a chronic toothache. She denied jaw pain, scalp tenderness, fevers, chills, weight loss and tinnitus.
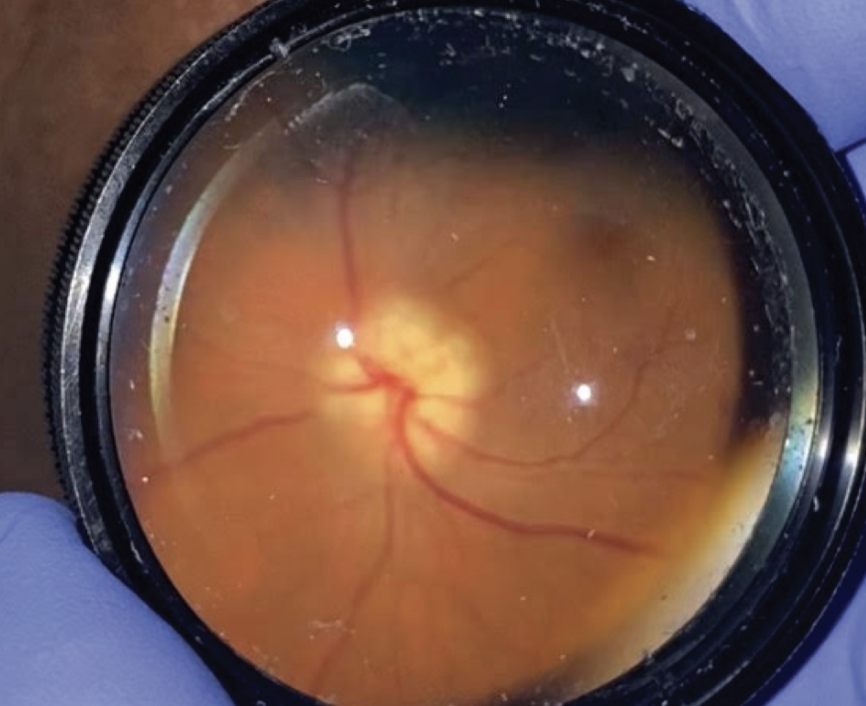 |
| Figure 1. Clinical photograph of the right optic nerve upon presentation showing optic nerve edema. |
Medical History
The patient’s past medical history was significant for type 2 diabetes mellitus, hypertension and hyperlipidemia. The patient recalled a questionable history of glaucoma. Her past surgical history included a cholecystectomy. She was currently taking carvedilol, irbesartan, aspirin and metformin. The patient was a non-smoker, denied drug use, and had a family history of type 2 diabetes mellitus and heart disease.
Examination
Upon physical exam, the patient’s vital signs were within normal limits. The eye examination revealed a visual acuity of hand motion OD and 20/40 pinhole to 20/25 OS. Pupils were 6 mm bilaterally with a 2+ relative afferent pupillary defect in the right eye. Intraocular pressure was 16 mmHg OD and 20 mmHg OS. By confrontation visual fields, she exhibited globally depressed visual fields in the right eye and full visual fields in the left eye. Her extraocular motility was full bilaterally. Examination with Ishihara color plates revealed 0/8 OD and 8/8 OS.
Anterior slit lamp examination of both eyes was unremarkable with the exception of 2+ nuclear sclerosis bilaterally. On dilated exam, the right optic nerve was found to have prominent disc edema and pallor (Figure 1). The left optic nerve had a cup-to-disc ratio of 0.7 with a healthy appearing rim. The rest of her fundus examination was unremarkable.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
Based on the findings, a differential diagnosis for severe, unilateral vision loss with associated optic nerve edema was considered. Included in this differential were arteritic anterior ischemic optic neuropathy, non-arteritic ischemic optic neuropathy, meningioma, infiltrative neoplasm, sarcoidosis, Lyme disease, tuberculosis, syphilis and fungal infection. The initial lab work-up included the following: erythrocyte sedimentation rate; C-reactive protein; complete blood count with differential; anti-myelin oligodendrocyte antibody; anti-aquaporin-4 antibody; ACE level; syphilis reverse sequence screening; ANCA antibody panel; ANA panel; IgG4 level; Lyme serologies; and interferon-gamma release assay.
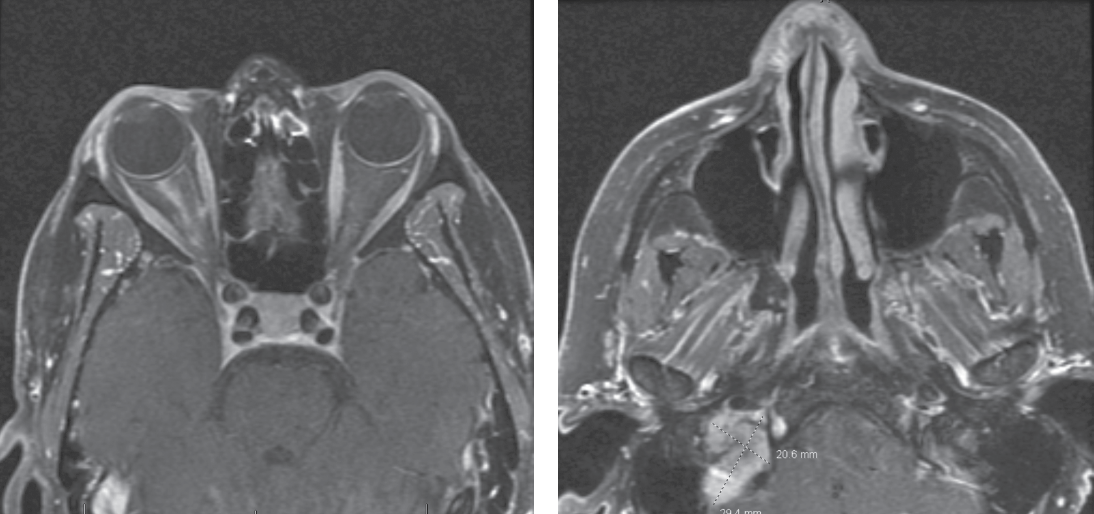 |
| Figure 2 (left). T1-weighted post-contrast MRI showing linear enhancement of the right optic nerve sheath complex. Figure 3 (right). T1-weighted post-contrast MRI showing isointense mass in the right jugular foramen. |
The patient underwent MRI brain and orbits with and without contrast, MRA head without contrast, orbital Doppler ultrasonography and chest X-ray. Her imaging revealed right peripheral optic nerve sheath enhancement (Figure 2). This finding was most compatible with infectious, inflammatory and vasculitic etiologies, although meningioma was also considered as a possibility. Imaging also revealed a right jugular foramen mass, most consistent with glomus jugulare tumor (Figure 3), as well as right corona radiata and internal capsular subacute infarcts. MRA revealed no flow abnormalities and no visualized aneurysms.
Orbital Dopplers revealed an elevated right disc with no vascular flow abnormalities. The ESR was 38, CRP was 0.80, and platelet count was 393, all of which were within the normal range when adjusted for age and gender. The remainder of her laboratory workup detailed above was within normal limits.
This patient’s history and examination were most concerning for arteritic anterior ischemic optic neuropathy, though the diagnosis was confounded by the presence of an ipsilateral glomus jugulare lesion and normal inflammatory markers. Otolaryngology was consulted for evaluation of the glomus jugulare lesion, which they assessed to be benign and chronic. The patient was admitted for stroke work-up and started on pulse-dose steroids with 250 mg of methylprednisolone every six hours. A temporal artery biopsy was performed, and the pathology was consistent with a diagnosis of giant cell arteritis. The patient completed five days of pulse-dose steroids and was discharged on 80 mg of oral prednisone.
On follow-up exam four weeks later, there was light perception vision in the right eye and stable vision in the left eye. Funduscopic examination of the right eye revealed a cup-to-disc ratio of 0.99, pallor and a thin rim circumferentially. The remainder of her eye examination was unchanged. Ocular coherence tomography revealed severe thinning of retinal nerve fiber layer and ganglion cell layer in the right eye, with normal thickness of the left eye (Figures 4 and 5).
Discussion
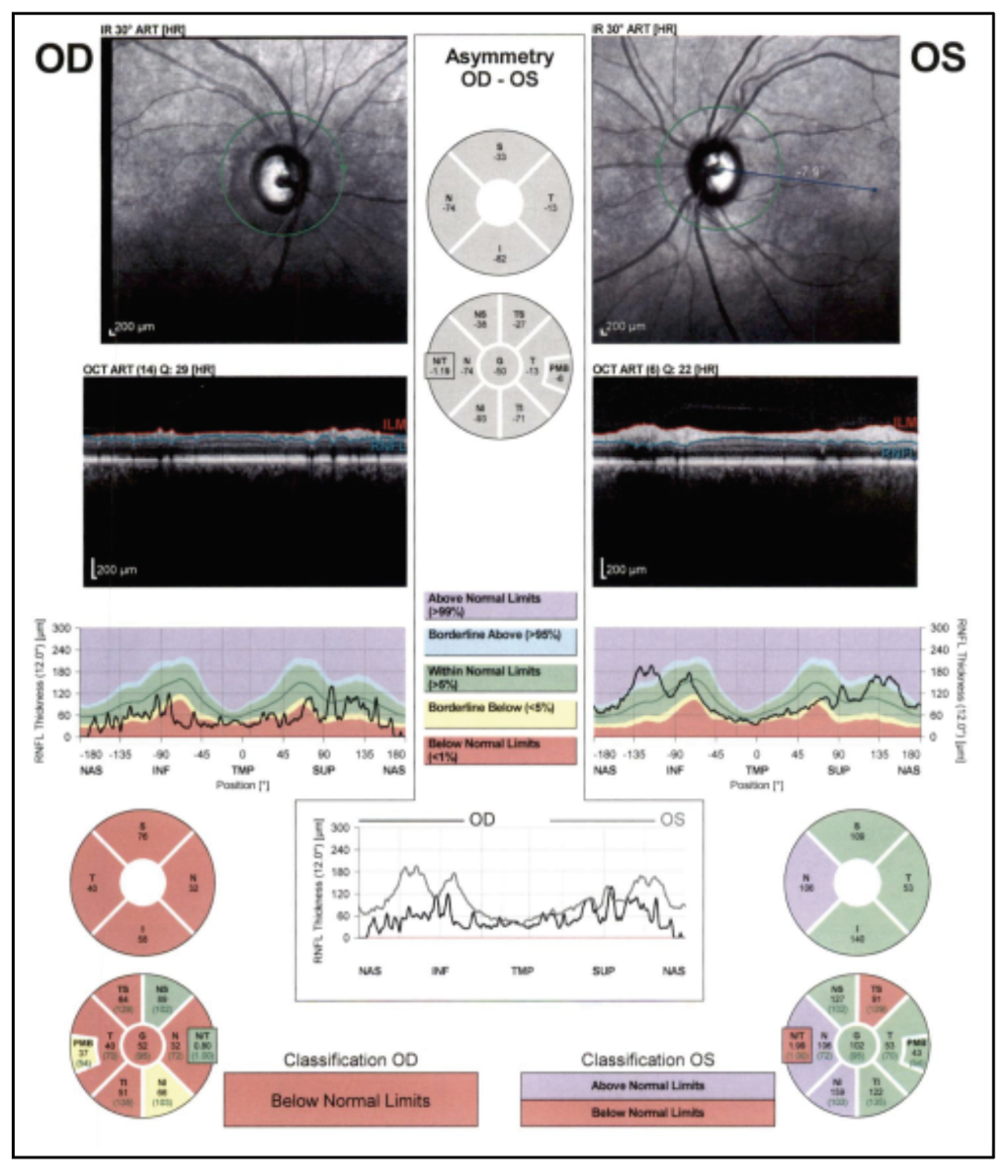 |
| Figure 4. OCT RNFL of both eyes, showing diffuse thinning of the right eye. |
This case highlights some key GCA take-home points:
• Definition and epidemiology. Anterior ischemic optic neuropathy is defined as “ischemia to the anterior part of the optic nerve, which is supplied by the posterior ciliary artery circulation.”1 Arteritic anterior ischemic optic neuropathy is defined as a vasculitis predominantly caused by Giant Cell Arteritis.2 GCA was first described in 1932 and is currently defined as granulomatous arteritis that predominantly affects the aorta and/or its major branches, predominantly the carotid and vertebral arteries, often involving the temporal artery.3,4 A recent meta-analysis estimates the pooled prevalence of GCA at 51.74 cases per 100,000 and the incidence at 10 cases per 100,000 for patients over 50, with those from Scandinavia disproportionately affected.5 With an incidence of 4.62 per 100,000 among Africans, our patient’s racial demographic group placed her at lower statistical risk for GCA; however, it’s imperative to evaluate all patients with signs of GCA, regardless of race.5,6
• Pathogenesis. GCA is a vasculitis that affects medium and large-sized arteries.2 It’s a result of T-cell and macrophage activation, resulting in a granulomatous pan-arteritis with intimal hyperplasia and occlusion of the vascular lumen.7 The ophthalmic manifestations of GCA are numerous and include AAION, APION, amaurosis fugax, retinal artery occlusion, cotton-wool spots, choroidal ischemia, anterior segment ischemia, extraocular muscle ischemia and motility disorders; ocular ischemic syndrome, and orbital inflammatory syndrome, with AAION being the most common.8,9
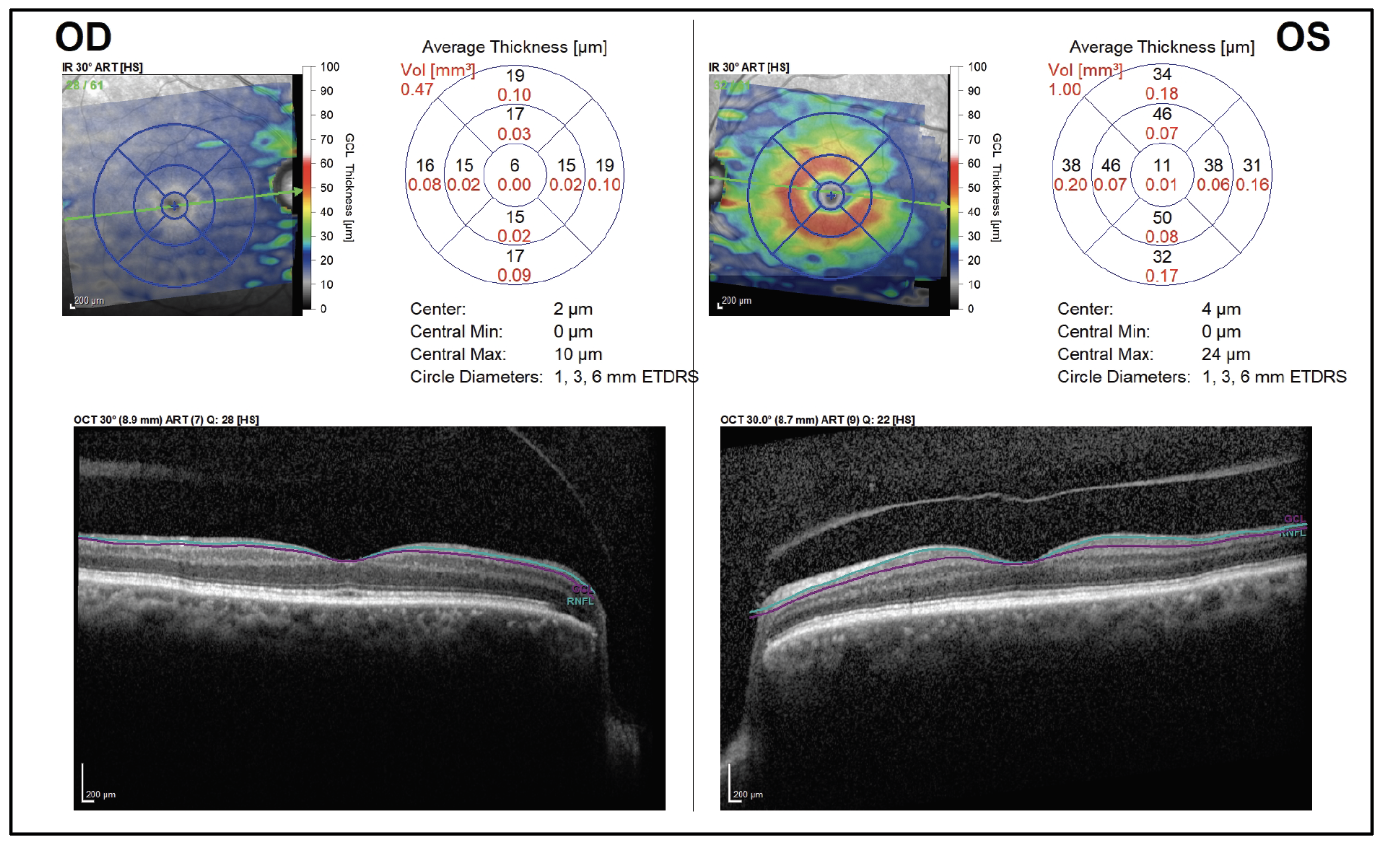 Figure 5. Ganglion cell analysis shows profound GCL loss of the right eye. |
• Diagnosis. The 2022 American College of Rheumatology/EULAR classification for GCA requires patients to be older than 50, and includes the following symptoms and diagnostic criteria: morning stiffness in the shoulders and/or neck; sudden visual loss; jaw/tongue claudication; temporal headache; scalp tenderness; elevated ESR; elevated CRP; positive temporal artery biopsy; bilateral axillary involvement; and FDG-PET activity throughout the aorta.10 The importance of the recommendation for TAB is underscored in this clinical case, as the diagnosis of GCA wouldn’t have been definitively made without a positive biopsy based on the ACR criteria.11
Ultrasound has been shown to be a useful adjunct in diagnosis, with elevated resistance on color duplex ultrasound of the temporal PCAs having a reported sensitivity of 86 percent and a specificity of 96 percent, with elevated resistance within the nasal PCA performing similarly well.12 In our case, orbital ultrasound was read as normal, highlighting the importance of maintaining a high clinical suspicion for GCA in cases where the history and clinical examination are concerning. A recent prospective study evaluating the sensitivity of color Doppler ultrasound of the temporal artery places the sensitivity much lower than orbital dopplers, at 5.1 to 30.8 percent.13
Reaching the diagnosis of GCA for this patient was complicated by the discovery of an incidental skull-base lesion and the presence of normal inflammatory labs. The sensitivity of a positive TAB for an elevated ESR and CRP is 86.9 and 84.1 percent, respectively. Only 4 percent of patients with normal ESR and CRP had a positive TAB for GCA.14 Our patient falls into this category, as the team’s clinical suspicion for GCA was high and a TAB was pursued despite normal inflammatory labs.
• Treatment and clinical course. It’s currently recommended to immediately treat suspected GCA with high dose glucocorticoids. Among those with acute or intermittent visual loss, it’s recommended to initiate pulse-dose, intravenous methylprednisolone.15 Studies place the risk of relapse during the course of GCA treatment between 40 to 79 percent.16,17 Continued monitoring of patients with GCA is important, given the risk of relapse and further vascular complications, though the utility and timing of imaging and laboratory markers aren’t well-established.18
In summary, a 74-year-old woman presented with a clinical picture concerning for GCA given her severe, unilateral vision loss and optic nerve edema with pallor. The treatment team maintained a high clinical suspicion for GCA despite normal inflammatory markers and orbital duplex ultrasonography. MRI of the brain and orbits and a temporal artery biopsy proved useful diagnostic techniques to confirm the diagnosis of GCA.
1. Hayreh SS. Anterior ischaemic optic neuropathy. Terminology and pathogenesis. Br J Ophthalmol 1974;58:12:955-963.
2. Hayreh SS. Ischemic optic neuropathy. Progress in Retinal and Eye Research 2009;28:1:34-62.
3. Horton BT, Magath TB, Brown GE. An undescribed form of arteritis of temporal vessels. Mayo Clin Proc 1932;7:700.
4. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. doi:10.1002/art.37715
5. Li KJ, Semenov D, Turk M, Pope J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther 2021;23:82.
6. Garrity ST, Pistilli M, Vaphiades MS, et al. Ophthalmic presentation of giant cell arteritis in African-Americans. Eye (Lond) 2017;31:1:113-118.
7. Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003;349:2:160-169.
8. Hayreh SS. Giant cell arteritis: Its ophthalmic manifestations. Indian J Ophthalmol 2021;69:2:227-235.
9. McFadzean RM. Ischemic optic neuropathy and giant cell arteritis. Curr Opin Ophthalmol 1998;9:6:10-17.
10. Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR classification criteria for giant cell arteritis. Annals of the Rheumatic Diseases 2022;81:12:1647-1653.
11. Murchison AP, Gilbert ME, Bilyk JR, et al. Validity of the American College of Rheumatology criteria for the diagnosis of giant cell arteritis. American Journal of Ophthalmology 2012;154:4:722-729.
12. Jianu D, Jianu N, Munteanu M, Petrica L. Clinical and ultrasonographic features in anterior ischemic optic neuropathy. VSP 2018;75:8:773-779.
13. Bilyk JR, Murchison AP, Leiby BT, et al. The utility of color duplex ultrasonography in the diagnosis of giant cell arteritis: A prospective, masked study. Trans Am Ophthalmol Soc 2017;115:T9.
14. Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum 2012;41:6:866-871.
15. Mackie SL, Dejaco C, Appenzeller S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology 2020;59:3:e1-e23.
16. Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: A retrospective cohort study. Rheumatology (Oxford) 2016;55:2:347-356.
17. Martinez-Lado L, Calviño-Díaz C, Piñeiro A, et al. Relapses and recurrences in giant cell arteritis: A population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore) 2011;90:3:186-193.
18. Koster MJ, Matteson EL, Warrington KJ. Large-vessel giant cell arteritis: Diagnosis, monitoring and management. Rheumatology (Oxford) 2018;57(suppl_2):ii32-ii42.



