Injectable botulinum toxin (BT) has a variety of medical and cosmetic uses. BT causes temporarily paralysis of muscles and therefore is effective for dynamic wrinkles, i.e., the wrinkles that develop during facial expression and movement. Long-standing static rhytids, which are present when the face is at rest, will be minimally affected. When assessing the patient, a helpful starting point is a hand-held mirror and the patient’s own analysis. Often they will request BT in regions that clearly will not benefit their perceived cosmetic problems; as with all therapies, good communication is paramount. Often the combination of BT and injectable fillers will be beneficial. It is important to counsel patients that after the first treatment session there will almost certainly be a touch-up session; subsequent treatments should be more reliable, as the dosing is recorded and can be referenced during follow-up visits. Nonetheless, due to variability in product and technique, touch-up sessions are not infrequent.
Current Products & FDA Labeling
Injectable botulinum toxin A (onabotulinumtoxinA; Botox; Allergan) was granted Food and Drug Administration approval in 1989 for the treatment of blepharospasm and strabismus. Botox Cosmetic (Allergan) received approval in 2002 for the treatment of glabellar rhytids, which to this day remains the only FDA-approved cosmetic indication for injectable botulinum toxin; notwithstanding, it is widely used for the treatment of forehead rhytids and periocular rhytids (“crow’s feet”), among others. Recently, other formulations of BT have been approved for cosmetic use: abobotulinumtoxinA (Dysport; Ipsen Biopharm Ltd.; Medicis Aesthetics Inc.) and incobotulinumtoxinA (Xeomin; Merz Pharmaceuticals). It should be noted that rimabotulinumtoxinB (Myobloc) is not FDA-approved for cosmetic use.
MOA and Contraindications
|
Any patient with a preexisting disorder of neuromuscular transmission should not receive BT, including patients with myasthenia gravis, Lambert-Eaton disease, amyotrophic lateral sclerosis and other disorders of the neuromuscular junction. BT should not be administered in patients who are pregnant, breast-feeding or currently undergoing systemic treatment with aminoglycoside antibiotics or other agents that interfere with neuromuscular transmission.
Preparations, Product Handling
BT is packaged in sterile, single-use vials, typically containing 100 units per vial (for Botox and Xeomin). The product itself is a rather delicate protein, so care is taken during the reconstitution process and in drawing up the toxin in syringes; overly rapid reconstitution or tapping the syringe to release air bubbles should be avoided. Some clinicians think that the fragility of BT has been overstated. Nevertheless, I still tend to handle the product gently.
Product labeling suggests that Botox should be used within 24 hours after reconstitution, although one study suggests that equivalent potency is retained up to six weeks after reconstitution when utilizing proper storage. Xeomin has the benefit of not requiring refrigeration prior to reconstitution. One theoretical benefit of Xeomin is that it is produced with fewer complexing proteins and thus may be less immunogenic. While the potency units for Xeomin are technically distinct from the potency units of Botox, their clinical dosing appears to be identical. Dysport, once reconstituted, is recommended by the manufacturer to be used within four hours. One “unit” of Botox is perhaps 2.5 to three times more potent than one “unit” of Dysport. Table 1 lists relevant features and product information for the various forms of injectable BT.
Reconstitution
|
Injection Technique, Patient Prep
Using a topical anesthetic can help to put a patient at ease and make the treatment process more enjoyable for the patient and the clinician. An effective topical anesthetic is benzocaine (40%)/lidocaine (6%)/tetracaine (4%) topical cream, left in place for at least 20 minutes. Simply using an ice cube for several seconds prior to injection can also be effective. Many patients, myself included, do not require anesthetic in the forehead, crow’s feet and glabella regions, however, nearly all patients require some form of anesthetic in the lip region. After providing suitable time for the topical anesthetic to work, the skin is gently cleansed of residual anesthetic cream and debris with an alcohol pad.
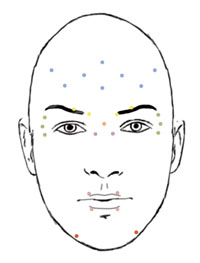
The BT injection pattern, discussed in the following section, is marked on the patient with a pen or makeup pencil. A sample injection pattern is presented in Figure 2. Digital pressure immediately after injection can reduce the risk of bruising, though that risk is low to begin with. Injections in the region of the corrugators usually lead to somewhat brisker bleeding than at other sites.
After injection, the patient will usually have some erythema at the injection that fades over 15 to 30 minutes. The patient should begin to see the effects of the BT in one to seven days. A touch-up session is scheduled for 10 to 14 days to make sure that the BT has had time to reach its peak effect before reinjection.
Aesthetic Evaluation & Dosage
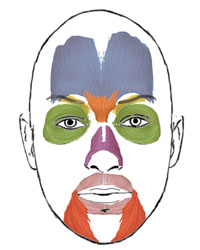
While it is simpler to discuss each muscle group for BT injection individually, it is important to consider their interplay in shaping important facial elements, such as the brow or lips. For example, brow position is affected by the combined actions of the corrugators, frontalis and
orbicularis. In addition, there are important gender differences to consider when determining an injection pattern near the brow; women tend to have a more arched brow than the flatter brow of men. The facial musculature of men is bulkier than that of women. When injecting the frontalis muscle, men may require 20 to 30 units, while women typically require only 10 to 20 units. The target muscles for facial BT injection are diagrammed in Figure 1.
| |||||||||||||||||||||||||||||||||
• Forehead. When approaching the forehead, the most important considerations involve brow position, as well as the rhytids near the brow. Injections that are performed too close to the brow (within 1 to 2 cm) can lead to brow ptosis or a “frozen” brow. Low lateral injections above the brow are to be avoided for this reason. However, injections that are too far above the tail of the brow can lead to the tell-tale isolated wrinkle that sits right above the lateral brow. Fortunately, by choosing a starting dosage that is relatively low and accepting a touch-up session, a stepwise and safe approach can be realized (See Table 2 and Figure 2).
One helpful technique to minimize unnatural segmental or lateral arching of the brow is to place the index finger firmly against the proposed injection site above the center of the brow. Doing so, and instructing the patient to raise their eyebrows, has the net effect of “simulating” BT. Splinting the medial and central brow digitally will help the clinician to identify the proper location of the lateral brow injection, relative to the midline.
• Glabella. The musculature of the glabella is comprised of the paired corrugator supercillii muscles (which generate the vertical glabellar furrows) and the procerus muscle (which generates midline horizontal furrows above the bridge of the nose). Injecting the corrugators can also offset some of the unwanted effects on brow position from frontalis injection. Overinjecting the muscles can lead to a “splayed” brow appearance, in which the paralysis causes the medial heads of the brow to be separated by an unnaturally large distance.
The corrugators can be grasped between thumb and forefinger. By having the patient activate their corrugators (making a “mad” expression), the lateral extent of the muscles can be appreciated. Typically a minimum of four corrugator injections are required, two evenly spaced injections on each side.
The injections are performed just above the brow cilia (See Figure 2), and should be relatively deep, to avoid frontalis involvement. Care must be taken to avoid direct injection into the supraorbital notch or foramen, which some clinicians think may be responsible for the occasional case of blepharoptosis after injection. (Happily, most cases of ptosis resolve in about two weeks, while the beneficial results of BT persist.) An additional injection into the procerus is usually required (See Table 2 and Figure 2).
|
• Crow’s feet. While most facial BT injections are deep, the orbicularis should be injected superficially to avoid bruising. These injections (See Table 2 and Figure 2) can shape the brow, and in women, can help to achieve a laterally arched brow. These injections should be performed at least 1 cm lateral to the lateral orbital inlet to avoid diffusion into the orbit.
Ophthalmologists are well-attuned to the functional role of the orbicularis in eyelid closure. Any BT injections in the orbicularis should be conservative in patients who have undergone recent blepharoplasty or refractive corneal surgery. In patients with lax lower eyelids, care must be taken to avoid lower eyelid ectropion; these patients may not be good candidates for injectable BT.
• Bunny Lines. Some patients complain of vertical lines adjacent to the nasal bridge—these are due to overaction of the transverse portion of the nasalis muscle. Usually a single injection on each side will resolve those rhytids (See Table 2 and Figure 2). Injections that are placed too inferior may risk involvement of the levator labii alaeque nasi and levator labii superioris muscles, which may lead to unwanted lip droop.
|
• Lip Region. Injecting the orbicularis oris (See Figure 1) with BT targets the radial perioral rhytids formed during lip puckering, such as those seen in smokers. Injections in the orbicularis oris should be superficial and close to the vermilion border. The injection pattern should be symmetric, and dosing should be low (See Table 2 and Figure 2). Placing injections significantly higher than the vermilion border can lead to lip ptosis, inversion or eversion. Avoid treating the corners of the lips, which could result in drooping and drooling, and avoid the midline of the upper lip, which will give it a flattened appearance. Keep in mind that the lips are quite sensitive, and extra time with application of ice prior to injection is warranted.
|
• Platysma. Platysmal bands are treated rather easily with spaced out injections along each band (See Table 2).
Other Considerations
After several injections, there will be a small amount of residual product in the vial that is not accessible by the needle. A bottle decapper (20 mm Kebby Decapper; Kebby Industries) is used to pry off the top of the vial to get the remaining product (See Figure 4), which is usually several units.
Manufacturer rebates are available from Allergan (
botoxcosmetic.com/home.aspx) and Merz Pharmaceuticals (
xeominaesthetic.com). In addition, both have incentives programs for patients who use their products. It is helpful to check their websites for up-to-date information. REVIEW
Dr. Walrath is an ophthalmologist and oculoplastic surgeon practicing with Paces Plastic Surgery and the Woolfson Eye Institute. He has no conflicts of interest to report for any products discussed in this review. Contact him at (404)-662-2266 or
drwalrath@josephwalrathmd.com.
1. Carruthers J, Fagien S, Matarasso SL, and the Botox Consensus Group. Consensus Recommendations on the Use of Botulinum Toxin Type A in Facial Aesthetics. Plast Reconstr Surg 2004;114 (Suppl): 1S.
2. Dressler D, Mander G, Fink K. Measuring the potency labeling of onabotulinumtoxinA and incobotulinumtoxinA in an LD50 assay. J Neural Transm 2012;119:13-5. Epub 2011 Oct 5.
3. Hexsel DM, Trindade de Almeida AT, Rutowitsch M., et al. Multicenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatol Surg 2003;29:523.
4. Trindade de Almeida AR, Kadunc BV, Di Chiacchio N, Neto DR. Foam during reconstitution does not affect the potency of botulinum toxin type A. Dermatol Surg 2003;29:530.