|
Overall, IOIS is the third most common orbital disorder, behind thyroid eye disease and lymphoproliferative disease, but perhaps due to its heterogeneous presentation and response to treatment, large-scale clinical studies are lacking, and our understanding of the disease process is still evolving.
Clinical Diagnosis
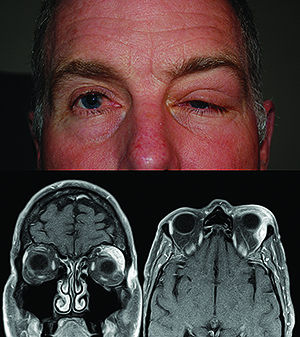
The classic presentation of IOIS includes the abrupt onset, often over the course of hours, of periorbital pain associated with edema, erythema and chemosis. Other common features include proptosis, diplopia and visual changes. A thorough workup is essential in all patients presenting with these orbital signs, beginning with a detailed review of systems, a full ophthalmic exam and dedicated orbital imaging. Although CT is often more readily available, MRI with and without gadolinium characterizes the location and extent of inflammation more accurately. Baseline blood work and serologic studies for patients with suspected IOIS may include a complete blood count with differential; erythrocyte sedimentation rate; C-reactive protein; angiotensin converting enzyme; cytoplasmic antineutrophil antibody; antinuclear antibody; rapid plasma reagin; thyroid function studies; and possibly a Lyme titer in specific patients living in endemic areas. While these studies cannot “rule in” IOIS, they may be helpful in ruling out other entities.
Beyond this initial workup, complete agreement on management is not currently available. One of the biggest hurdles to consensus to the understanding of IOIS is what, precisely, is the diagnosis? The inflammatory signs and symptoms of IOIS (pain, chemosis, erythema, etc.) most likely represent the clinical manifestations of a variety of autoimmune and cell-mediated processes.2 But is “inflammation” a valid diagnosis, or is it merely a tissue response related to some other process3 either not yet described by medicine or not yet elucidated by available testing?
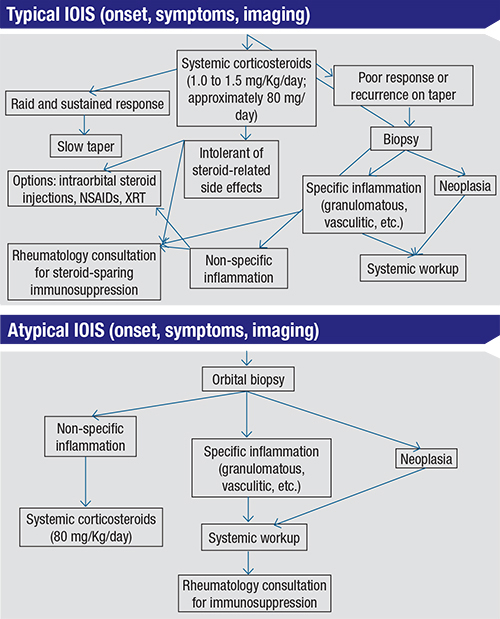
This controversy divides clinicians broadly among two camps. Some consider the typical clinical presentation, when supported by positive findings on appropriate imaging studies and without another attributable cause, to be almost diagnostic. A “corticosteroid trial” followed by the rapid and complete resolution of a patient’s signs and symptoms helps to support the diagnosis. Other experts believe that an orbital biopsy should be attempted in all patients prior to the initiation of steroid treatment of potential IOIS, provided the tissue in question is easily accessible.3 They argue that “inflammation” is not a diagnosis, but may be a sign of a potentially dangerous underlying tissue process.
 |
Pathology
Pathologically, the most common description of IOIS is that of “non-specific inflammation.” Histology most often demonstrates a paucicellular infiltrate of lymphocytes, plasma cells and histiocytes. Particular cases may, however, show more specific patterns such as lymphoid follicles with germinal centers, destruction of normal tissue structures, granulomas and sclerosis. In his 2005 ASOPRS Foundation Lecture, Gerald J. Harris, MD, delineated a pathologic construct for IOIS,2 helping to improve our understanding of the disease process (and its variations in both clinical practice and as seen in pathologic specimens) by correlating the specific cells present in surgical specimens with particular clinical symptoms. For example, macrophages activated by pro-inflammatory cytokines cause lysis of cells, escalating a cascade of inflammation and causing tissue injury and fibrosis which can result in the clinical symptoms of orbital pain, swelling and impaired function.2
This insight into the “pathologic construct,” coupled with improving pathologic abilities to identify and measure cytokine levels and activities, has opened the door to further research into specific inflammatory mediators involved in IOIS. One such study demonstrates that gamma interferon and interleukin-12 levels are markedly elevated in IOIS when compared to normal, non-inflamed tissue profiles.10 More studies will likely follow, the results of which will not only contribute to our understanding of the pathology of IOIS, but also may identify targets for treatment with therapeutic biologic agents.
Management
The goal of treatment in all cases of IOIS is to intercept the systemic inflammatory cascade in order to halt its progression, prevent permanent damage to the orbital contents and reduce patient discomfort. Typically the treatment algorithm begins with the use of oral or parenteral corticosteroids (starting oral dose should be between 1.0 and 1.5 mg/kg/day, or approximately 80 mg of prednisone a day for a 70 kg adult) and/or NSAIDs.2 Some clinicians advocate the use of intralesional steroid injections.5 In patients with steroid-sensitive disease who are intolerant of steroid-related side effects, orbital radiation has been used with some success.6 Neither of these treatment modalities differentiates between specific and non-specific types of inflammation, however. Side effects of prolonged steroid treatment are well-known and include electrolyte imbalances; osteoporosis; elevated serum glucose levels; sleep disturbances; and mood disorders, among others. Ocular complications as a result of radiation therapy are rare but not insignificant, and include keratoconjunctivitis, cataract formation and retinopathy.
Immunosuppressive agents, including anti-metabolites (e.g., methotrexate, azathioprine), alkylating agents (e.g., cyclophosphamide, chlorambucil) and T-cell inhibitors (e.g., cyclosporine, tacrolimus), are increasingly used as steroid-sparing agents.2,4,7,8 These medications may be better-tolerated than corticosteroids in some patients; however, they are equally non-specific, and often require ongoing serologic monitoring and close clinical follow-up.
An increasing understanding of the immunopathology of IOIS may improve our ability to target more specific elements of the inflammatory cascade, reducing co-morbidities associated with global immunosuppression. This has lead to a trend towards the use of immunomodulators that target specific cytokines that mediate the inflammatory response, such as tumor necrosis factor-alpha (TNF-a), (e.g., infliximab).9 The recent discovery of elevated levels of gamma interferon, TNF-a, and interleukin-12 in biopsy-proven IOIS may improve our ability to more specifically treat this process in the future.10
Because the initial diagnosis of IOIS is often made on clinical grounds without tissue verification, a period of prolonged observation after the resolution of a patient’s acute symptoms is advisable. Although recurrences are possible, they are not the norm. A recurrence of inflammation in the same or different anatomic location should arouse suspicion, and may prompt biopsy to rule out masquerade syndromes. Having a very candid conversation with patients about this possibility at initial diagnosis can help to ensure cooperation with this prolonged surveillance.
That the diagnosis of IOIS remains a challenging subject despite our growing understanding of the molecular basis of disease should invoke a certain level of caution in the treating physician. A brief review of the history of IOIS will reveal a diagnosis in transition over the past half century; patients once categorized under the umbrella term of “orbital pseudotumor” may now be diagnosed with sarcoidosis or Wegener’s granulomatosis. It is not unreasonable to assume that future serologic tests may further whittle down this group now classified as idiopathic. REVIEW
Dr. Lane is in private practice in Burlington, Vt. Contact her at: katealane@gmail.com.
1. Weber AL, Jakobiec FA, Sabates NR. Pseudotumor of the orbit. Neuroimaging Clin North Am 1996;6:73-91.
2. Harris G. Idiopathic Orbital Inflammation: A Pathogenetic Construct and Treatment Strategy. Ophthal Plast Reconst Surg 2006;22:79-86.
3. Rose GE. A Personal View: Probability in Medicine, Levels of (Un)Certainty, and the Diagnosis of Orbital Disease (With Particular Reference to Orbital “Pseudotumor”. Arch Ophthalmol 2007;125:1171-2.
4. Mombaerts I, Goldschmeding R, Schlingemann RO, Koornneef L. What is orbital pseudotumor? A clinical pathological review. Surv Ophthalmol 1996;41:66-78.
5. Leibovitch I, Prabhakaran VC, Davis G, Selva, D. Intraorbital injection of triamcinolone acetonide in patients with idiopathic orbital inflammation. Arch Ophthalmol 2007;125:1647-51.
6. Smitt MC, Donaldson SS. Radiation therapy for benign disease of the orbit. Semin Radiat Oncol 1999;9:179-89.
7. Yuen SJ, Rubin PA. Idiopathic orbital inflammation: Distributions, clinical features and treatment outcome. Arch Ophthalmol 2003;121:491-9.
8. Rubin PA, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol 2004;138:1041-3.
9. Garrity JA, Coleman AW, Matteson EL, et al. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol 2004;138:925-30.
10. Wladis EJ, Iglesias BV, Gosselin EJ. Characterization of the molecular biologic milieu of idiopathic orbital inflammation. Ophthal Plast Reconst Surg 2011;27:251-4.