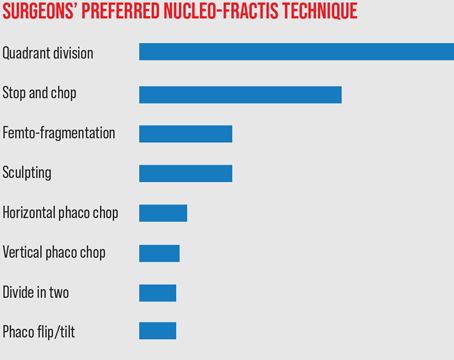
Cataract surgery is the most frequently performed surgery in the world, but it can be complicated by many factors—including a coexisting disease such as glaucoma. In that situation, the cataract surgeon needs to make a number of adjustments to ensure a good outcome, especially if the patient has advanced glaucoma.
Here, I’d like to discuss eight things you can do to ensure the best possible outcome when performing cataract surgery in this circumstance.
1. Do careful surgical planning. This means answering several questions ahead of time:
— If the advanced glaucoma is uncontrolled, should you combine the glaucoma and cataract procedures or perform them separately? If the advanced glaucoma isn’t well-controlled, you need to prioritize the glaucoma surgery. That means either doing the glaucoma surgery first and waiting to do the cataract surgery, or doing them both at the same time.
One advantage to combining the procedures is that doing so can simplify a filtering surgery. You might not need to do an iridectomy, and that might help to minimize postoperative inflammation. Also, placement of a tube in the sulcus can be much easier in a pseudophakic eye.
However, specific factors tied to the individual’s situation could make staging the surgeries a better option. If the patient has pseudoexfoliation, for example, the eye might have a small pupil and weak zonules. You might need to insert a capsular tension ring during the cataract removal, and there might be a high risk of vitreous loss. In that situation it might be preferable to avoid doing both surgeries at the same time. Also, a combined procedure wouldn’t be advisable if you hope to implant a toric lens, because a bleb or tube shunt can have an unpredictable effect on astigmatism.
However, if you decide to do the glaucoma surgery first and postpone the cataract surgery, two problems will arise. First, the glaucoma procedure is likely to accelerate cataract progression. Second, if you create a bleb, subsequent cataract extraction could lead to bleb failure. Studies have shown that the longer you wait between the trabeculectomy and the cataract surgery, the better the chances that the trabeculectomy bleb will survive the cataract surgery—but the longer you wait, the worse the cataract will become.
— If the glaucoma is well-con-trolled, should you add a MIGS procedure to the cataract surgery to reduce the patient’s medication burden? We generally think of MIGS surgery as being reserved for mild to moderate glaucoma, but in a patient with well-controlled advanced glaucoma, a MIGS procedure can de-crease the medication burden. If the patient is intolerant of some of the medications being taken, or simply wants to reduce the medication bur-den, it might make sense to consider MIGS surgery.
— Which type of intraocular lens is most likely to produce a good outcome? The vast majority of these
patients receive aspheric monofocal IOLs. If the patient has astigmatism, there’s some reason to consider implanting a toric IOL; they’ve been shown to improve vision in patients with advanced visual field loss.1
 |
However, there are several challenges in this situation. The first challenge is getting stable readings at the cornea in order to choose the appropriate IOL. (If the patient’s prior trabeculectomy has been stable for some time, you probably can get good measurements.) But if you’re thinking about doing a combined cataract and glaucoma surgery, there’s an even more daunting challenge: It’s very hard to predict what impact the trabeculectomy will have on the astigmatism. (MIGS surgeries are more astigmatically neutral, so a MIGS procedure might allow you to more accurately plan for a toric lens.)
Of course, multifocal IOLs are generally not advisable for anyone with advanced glaucoma; they under-cut contrast sensitivity, which is al-ready a problem for an advanced glaucoma patient.
— Is the patient truly going to benefit from cataract removal, or should you consider postponing the surgery? Even when an individual has significant damage from advanced glaucoma, it’s been shown that removing a cataract improves both best corrected visual acuity and quality of life. For example, a study in the Journal of Glaucoma looked at 93 patients with both cataract and advanced glaucoma.2 They measured pre- and postoperative vision-related quality of life, BCVA and weighted-average LogMAR vision. All subjects improved after cataract surgery, in terms of general vision, mobility, psychological adjustment, reading, fine work and activities of daily living.
Nevertheless, it’s not always clear that removing the cataract in a patient with advanced glaucoma will improve the patient’s vision. If the cataract is fairly advanced, it may be difficult to tell how much of the vision impairment is due to the cataract and how much is attributable to the glaucoma. This is an issue because cataract surgery is riskier in these patients, so you have to weigh the risks and benefits of doing the cataract surgery.
2. Manage expectations (your own and your patient’s). Make sure that both you and the patient have an accurate sense of what you can realistically do for him in this circumstance. (This should be reflected in the informed consent.) Issues include:
— You can only correct vision loss from the cataract, not from the glaucoma. The patient shouldn’t expect this procedure to restore the vision he or she had earlier in life.
— A postoperative IOP spike is possible. If your patients aren’t warned about this, they may believe you did something wrong when their IOP rises and needs to be managed.
— If patients have had a prior trabeculectomy, they need to understand that cataract surgery could affect the bleb. The patient should understand that bleb failure is possible, and that separating the cataract and glaucoma procedure could lessen the risk.
— A prior trabeculectomy patient should consent to your per-forming a bleb revision and/or antimetabolite injection. In some cases these could become necessary.
— Warn patients that the cataract removal may not result in a lower IOP. Given patients’ access to information today, they may expect the cataract surgery to lower their IOP and conclude you didn’t do the surgery properly if it fails to happen.
However, clinical data suggests that a pressure drop may not hap-pen in patients with advanced open-angle glaucoma. First of all, patients who are well-controlled or have a low pressure don’t generally have an IOP drop after cataract extraction.3 And, in patients with a prior trabeculectomy, the IOP may even increase because the cataract surgery can cause inflammation and scarring in the bleb.4 You should include this possibility in your discussion and informed consent.
3. Do what you can to prevent a postoperative IOP spike. Keep in mind all of the factors that can increase the likelihood of a post-operative IOP spike, and do what you can to mitigate them. Also, as noted earlier, include the possibility of a postoperative IOP spike in your discussion with the patient and in the informed consent.
Factors increasing the risk of a post-operative IOP spike include:
— The presence of glaucoma—especially pseudoexfoliation. Studies have found that patients with pre-existing glaucoma (especially pseudoexfoliation) tend to have a more complex cataract surgery, and are likely to have potentially harmful IOP spikes between three and seven hours after surgery.5,6 Another study found that even medically well-con-
trolled glaucoma patients often experienced IOP spikes.7
— The type of viscoelastic you use. Cohesive viscoelastic is less likely to cause an IOP spike than a dispersive viscoelastic, primarily because it’s easier to completely remove at the end of surgery. For example, one study found that Viscoat caused significantly higher IOP increases and significantly more IOP spikes (and higher-pressure spikes) than DuoVisc in the early postoperative period.8 Another study found that this was also true when comparing Viscoat to Healon 5 following small-incision cataract surgery.9
— A long axial length; a previous trabeculectomy or laser trabeculoplasty; and a greater number of glaucoma medications. These were associated with an in-creased risk of postoperative IOP spikes in a study of this topic conducted at the University of Washington.10 (The correlation to a greater number of preoperative glaucoma medications may be a surrogate for how advanced the patient’s disease was.)
A common way to lower the risk of a postoperative IOP spike is to give the patient a pressure-lowering drug before or after surgery. Agents used for IOP spike prophylaxis include oral acetazolamide, Miostat and topical anti-hypertensives, including combination drops such as CoSopt (Merck).7,11-13
It turns out that timing counts; when the prophylactic agent is given to the patient can make a big difference in how effective it is. A study done in 2017 compared the impact of timing when giving oral acetazolamide to 90 open-angle-glaucoma patients with moderate to advanced glaucoma with 90 eyes undergoing cataract surgery.14 The researchers ran-domized the groups to either one hour preoperatively, three hours post-operatively, or no medication at all. Then they measured the pressure at multiple time points to see how many eyes had a significant pressure spike, defined as more than 100 percent above the preoperative IOP.
They found that the patients receiving the drug one hour preoperatively did the best; they were least likely to reach that threshold. If the drug was given orally three hours after surgery, IOP elevation wasn’t reduced until five hours or more after surgery.
This is very practical information. Before I saw this paper I was giving my patients oral prophylaxis after the surgery, which this study showed is inferior to one hour preoperatively. Furthermore, I’d been using an extended-release tablet; the good outcomes in the study were achieved with an immediate-release tablet. I always found a good pressure when seeing the patient the following day; that led me to believe I’d prevented a pressure spike. But this study makes it clear that giving oral acetazolamide one hour preoperatively—using immediate-release tablets—is a better way to prevent IOP spikes four to six hours after surgery.
Note: If you’re combining cataract surgery with trabeculectomy, avoiding hypotony is also a concern. In that situation, you may want to plan for laser suture lysis after the first postoperative week. Hypotony will be less of an issue if the added surgery is a MIGS procedure, since the risk of hypotony is lower with MIGS.
| Cortex left behind has been associated with increased inflammation. |
4. Minimize inflammation. Two strategies can help ensure that postoperative inflammation is minimal. First, manipulating the iris will increase inflammation. So, to enlarge the small pupil, try using a bolus of intracameral lidocaine or a viscoelastic such as Healon 5. (Of course, if necessary, use whatever iris expansion technique you feel comfortable with.) Second, make sure you take the time to remove all of the cortex before completing the surgery. Cortex left behind has been associated with increased inflammation.
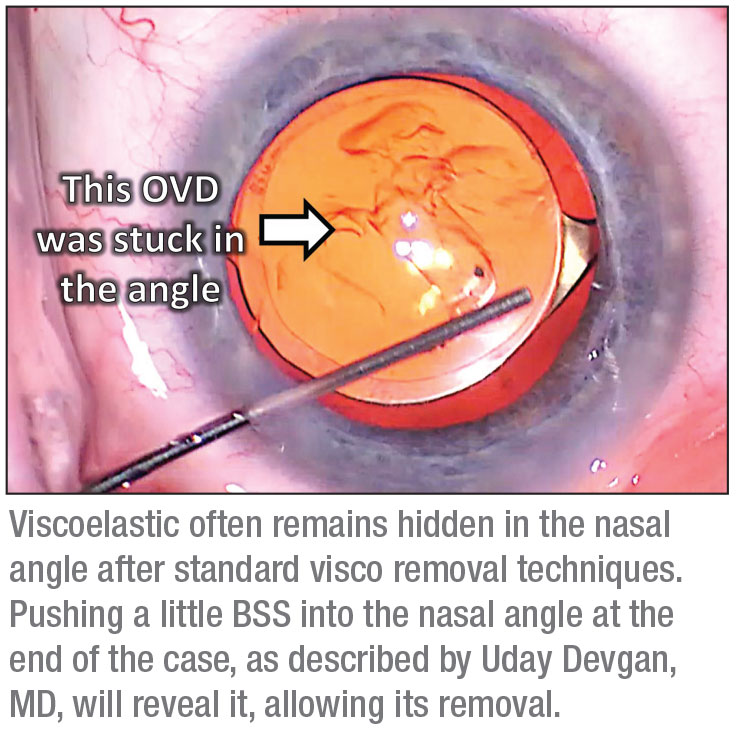
5. Remove all viscoelastic. Because you want to maintain the anterior chamber depth through-out the surgery—especially in hyperopic eyes and those with prior filtering surgery—you’re going to use a lot of viscoelastic. If the patient has had a prior filtering surgery, some of that viscoelastic may try to exit the eye through the filter, making it more challenging to maintain adequate anterior chamber depth during surgery. To ensure the best possible outcome, you need to remove all of that viscoelastic. It’s worth spending extra time to make sure you do—especially if the patient is a high myope. High myopes have larger eyes, so you’ve probably injected a substantial amount of viscoelastic, and it can be hidden.
To ensure you remove all of it, be sure to sweep behind the IOL. Then, carefully remove all the viscoelastic in the anterior chamber. Finally, do the “angle sweep” described by Uday Devgan, MD. The idea here is that when we inject viscoelastic into the eye at the beginning of the surgery, we tend to inject it into the nasal angle, because your cannula is coming in from the temporal incision. When performing the “angle sweep” the surgeon pushes a little BSS into the nasal angle at the end of the case. You’ll be surprised how much unseen viscoelastic will appear as a result of this maneuver. (See image, p. 55.)
You can see an example of this technique, and its results, at cataractcoach.com/2018/11/19/the-angle-sweep-technique-to-remove-viscoelastic/. In the video, it looks like Dr. Devgan has done a great job of removing all of the viscoelastic in the standard manner. Then he takes the cannula and injects some BSS into the nasal angle—and a significant amount of retained viscoelastic appears. That’s why the “angle sweep” is a technique that’s worth learning.
6. If the patient has had prior trabeculectomy, protect and/or revive the bleb. A number of maneuvers we make during cataract surgery could easily result in contact with an existing bleb. In order to avoid causing unintended problems, it pays to be conscious of this and go out of your way to avoid making contact.
Also, give some thought to where you place your paracentesis, especially when operating on a right eye. That’s because in a right eye with a trabeculectomy, the bleb may be positioned right where you’d normally make the paracentesis.
Finally, consider injecting a little subconjunctival 5FU at the end of the case (as long as there’s no contraindication). Performing surgery on the eye causes inflammatory metabolites to increase, and those will end up passing through the bleb. Injecting a little 5FU helps to prevent this from causing scarring. Studies have found that doing so has a protective effect on a functioning bleb and can be used routinely at the end of phacoemulsification in such cases.15 I usually inject it inferiorly, away from the bleb; it diffuses and has the effect on the bleb that I want. Some surgeons might inject it adjacent to the bleb; I just feel more comfortable injecting it 180 degrees away.
It’s possible that the bleb may need revision. To get a sense of how well the bleb is functioning—if you’re not sure—you can inject trypan blue into the eye. A 2018 study used this approach, grading the blebs as having mild or diffuse staining; at one year postop, there was a trend toward needing more IOP-lowering medications among those eyes that had only mild bleb staining, although the difference wasn’t statistically significant (p<0.10).16
If the bleb is working fine, I wouldn’t touch it. I’d just try to do the shortest, cleanest cataract surgery I can and then inject a little 5FU as de-scribed above. If bleb function is suboptimal, you can consider doing ab interno revision, or ab externo needling along with the 5FU injection.
7. Schedule frequent follow-up. Given the higher risk of a postoperative IOP spike, you may want to see these patients more often than a routine cataract patient, especially in the day or two following the surgery. This is especially important if the patient has had a prior trabeculectomy or tube shunt, to monitor for IOP issues and early signs of bleb failure. (All surgeons have their own preferences for scheduling follow-up visits, so there’s no specific formula that should be followed.)
One suggestion I haven’t yet tried is scheduling an advanced-glaucoma patient early in the day. This gives you the option of asking the patient to stay in the office or ASC for several hours, so you can easily do a same-day IOP check and treat if necessary.
8. Increase the frequency of steroids compared to a typical phaco case, and consider the use of postop antimetabolites. For a routine patient, I’d prescribe prednisone 1% four times a day postop, but if the patient has a prior trabeculectomy or tube surgery, I might have the patient use it hourly or every two hours initially, to make sure the bleb stays functional. I’d have the patient do that for the first week, followed by a gradual taper. I’d also consider additional subconjunctival injections of 5FU in patients with prior trabeculectomy, especially if the bleb isn’t functioning as it should be.
An Ounce of Prevention
Taking steps like these will help ensure that any cataract patient with advanced glaucoma avoids unwanted problems during surgery and isn’t disappointed because of unrealistic expectations postoperatively. That will mean a better outcome, a happier patient and less worry for you. REVIEW
Dr. Ou is an associate professor of ophthalmology, co-director of the glaucoma division
and vice chair for postgraduate education in the Department of Ophthalmology at the UCSF School of Medicine in San Francisco. She is a consultant for Merck.
1. Brown RH et al. (March 2017). Toric Intraocular Lens Outcomes in Glaucoma Patients with Advanced Visual Field Loss, 2017 American Glaucoma Society meeting, Coronado, CA.
2. Xu X, Sun Q, Ma YY, Zou HD. Vision-related quality of life outcomes of cataract surgery in advanced glaucoma patients. J Glaucoma 2016;25:1:e5-11.
3. Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg 2008;34:5:735-42.
4. Swamynathan K, Capistrano AP, Cantor LB, WuDunn D. Effect of temporal corneal phacoemulsification on intraocular pressure in eyes with prior trabeculectomy with an antimetabolite. Ophthalmology 2004;111:4:674-8.
5. Ahmed II, Kranemann C, Chipman M, Malam F. Revisiting early postoperative follow-up after phacoemulsification. J Cataract Refract Surg 2002;28:1:100-8.
6. McKellar MJ, Elder MJ. The early complications of cataract surgery: Is routine review of patients 1 week after cataract extraction necessary? Ophthalmology 2001;108:5:930-5.
7. Levkovitch-Verbin H, Habot-Wilner Z, Burla N, et al. Intraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or exfoliation syndrome. Ophthalmology 2008;115:1:104-8.
8. Rainer G, Stifter E, Luksch A, Menapace R. Comparison of the effect of Viscoat and DuoVisc on postoperative intraocular pressure after small-incision cataract surgery. J Cataract Refract Surg 2008;34:2:253-7.
9. Rainer G, Menapace R, Findl O, Georgopoulos M, Kiss B, Petternel V. Intraocular pressure after small incision cataract surgery with Healon5 and Viscoat. J Cataract Refract Surg 2000;26:2:271-6.
10. Slabaugh MA, Bojikian KD, Moore DB, Chen PP. Risk factors for acute postoperative intraocular pressure elevation after phacoemulsification in glaucoma patients. J Cataract Refract Surg 2014;40:4:538-44.
11. Solomon KD, Stewart WC, Hunt HH, Stewart JA, Cate EA. Intraoperative intracameral carbachol in phacoemulsification and posterior chamber lens implantation. Am J Ophthalmol 1998;125:1:36-43.
12. Cekiç O, Batman C. Effect of intracameral carbachol on intraocular pressure following clear corneal phacoemulsification. Eye (Lond) 1999;13(Pt 2):209-11.
13. Hayashi K, Yoshida M, Sato T, Manabe S. Effect of topical hypotensive medications for preventing intraocular pressure increase after cataract surgery in eyes with glaucoma. Am Journal Ophthalmol 2019;205:91-98.
14. Hayashi K, Yoshida M, Manabe SI, Yoshimura K. Prophylactic effect of oral acetazolamide against intraocular pressure elevation after cataract surgery in eyes with glaucoma. Ophthalmology 2017;124:5:701-708.
15. Sharma TK, Arora S, Corridan PG. Phacoemulsification in patients with previous trabeculectomy: role of 5-fluorouracil. Eye (Lond) 2007;21:6:780-3.
16. Yung ES, Moster MR, Sanvicente C, et al. Trypan blue for the assessment of filtering bleb function during cataract surgery. J Glaucoma 2018;27:3:246-250.