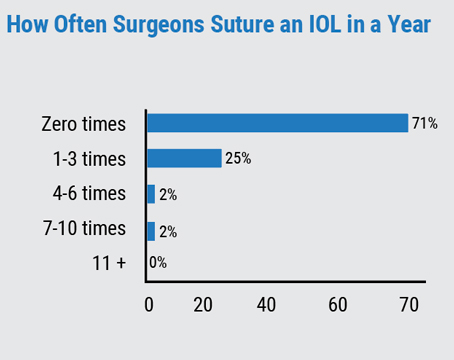
Calculating the best intraocular lens power for patients undergoing cataract surgery remains a key part of achieving patient satisfaction. But despite constant advances in biometry and ever-improving power-calculation formulas, most practices are still only getting about 80 percent of patients within 0.5 D of the target refraction. Here, experts explain how the measurement and calculation process has evolved, why it still has limits, and what lies ahead.
New Formulas, Better Outcomes
Surgeons now have access to an ever-increasing number of complex and highly accurate formulas. So: How should a surgeon proceed?
Kenneth J. Hoffer, MD, FACS, a clinical professor of ophthalmology at the Stein Eye Institute, University of California Los Angeles, points out that when it comes to using IOL power calculation formulas, there are two groups of ophthalmologists. “One group reads about the latest developments, stays up to date and goes to meetings,” he says. “They want to use the latest formulas. That group of doctors pretty much has moved to using the Barrett Universal II, Kane, EVO or other recent methods. The other group of ophthalmologists feels more comfortable with what they’ve always used. Of course, they’re not monitoring their outcomes. If they did, they’d realize they’ve fallen behind.
“In 2022, most of us don’t use the Hoffer Q, Holladay I or SRK/T anymore,” he continues. “Now we look at the formulas that have been developed in recent years. Their algorithms are secret, but not in a bad way; they’re so complicated that you can’t really publish a paper that describes exactly how they work, and most of them involve the use of artificial intelligence.
“Today,” he notes, “pretty much everybody understands that the Barrett Universal II is good, and many others are good as well, including the Kane formula; the EVO 2.0 formula from T.K. Yeo, MD, in Singapore; the RBF 3.0; and the Pearl-DGS formula developed by Damien Gatinel, MD, and Guillaume Debellemanière, MD, in France. The last formula was actually published, despite being complex and involving AI.1 It’s a good formula.”
Part of increasing the accuracy of this calculation is taking more factors into account—often, things that weren’t seen as important earlier. “For example, several current formulas now incorporate gender into the required data,” Dr. Hoffer says. “I incorporated both gender and race into a formula called the Hoffer H-5 some time ago, but it never caught on. More recently, Jack Kane, MD, incorporated gender into his Kane formula, which uses AI. Now it’s becoming accepted that gender is an important part of lens power calculation. The studies they did,2 as well as one done by Ronald Melles, MD,3 on a very large series of eyes showed the Kane formula to be even more accurate than the Barrett.”
Changing With the Times
Surgeons whose formulas first appeared several years ago have been motivated to update and improve the originals. Dr. Hoffer, and Warren E. Hill, MD, FACS, medical director of East Valley Ophthalmology in Mesa, Arizona, and developer of the Hill-RBF power calculation method, both recently released new versions of their formulas.
Dr. Hoffer says he realized a few years ago that the original Hoffer Q formula was outmoded. “Dr. Melles worked with Jack Holladay, MD, to conduct a study comparing the different formulas in thousands of eyes,” he explains. “The results made it clear that the Hoffer Q couldn’t compete with some of the newer formulas. At that point it had been around for more than 25 years, so I decided it was time to ask doctors to stop using it. However, many ophthalmologists stick to their old ways and keep using the formulas they’ve used for years. So, I finally decided that if I couldn’t get everyone to move on, the next best thing would be to update the formula to make it competitive again.
Calculation Evolution “The big story is that IOL power calculations are approaching a ceiling, in terms of their performance,” says Jack T. Holladay, MD, MSEE, FACS, the developer of the Holladay 1, 2 and Refractive Formulas, and president of Holladay Consulting. “They’re primarily limited by measurement error—axial length and corneal power—and prediction of the effective lens position. The improvements we’ve seen have become smaller and smaller over the years because we’ve improved the formulas about as much as we can. “Back in the 1980s, maybe 25 to 30 percent of patients ended up within half a diopter of target,” he says. “That was pretty good compared to the early days! But then we began to use vergence formulas with data from keratometry and immersion A-scan. “Prior to the 1990s, our axial length measurements were made with ultrasound,” he continues. “Ultrasound doesn’t actually measure to the retina and has some other problems as well. But around 1990 automated optical biometers using interferometry appeared, and our accuracy with this measurement improved from about ±0.3 mm—which translates to ±1 D in terms of outcomes— to about ±0.1 mm, which narrows the outcome error to ±0.25 D. The other thing that was important was that the automated keratometer on those instruments eliminated human error.” In the early 1990s, Kenneth J. Hoffer, MD, FACS, a clinical professor of ophthalmology at the Stein Eye Institute, University of California Los Angeles, who has been involved with biometry and lens calculation since 1974, was the first to publish evidence that different formulas produced better results in eyes with different axial lengths. “To see which formulas performed best, I looked at short eyes with an axial length less than 22 mm; very long eyes, greater than 26 mm; and medium-long eyes—24.5 to 26 mm,” he explains. “My Hoffer Q formula worked better in short eyes; the SRK/T worked better in long eyes, and Holladay I worked best in medium-long eyes. In average eyes they were pretty much equally accurate. So cataract surgeons became accustomed to choosing a lens power formula based on the axial length.” Dr. Holladay says that, by the 1990s, about 50 to 55 percent of cataract surgery patients were ending up within 0.5 D of target. Subsequent work by Doug Koch, MD, and Li Wang, MD, based on more accurate data for longer eyes, improved results about another 5 percent. “In the early 2000s, new formulas from Graham Barrett, MD, and Thomas Olsen, MD, were developed using the optical biometer,” Dr. Holladay notes. “Those formulas took our accuracy up to 65 or 70 percent. Next, Paul-Rolf Preussner, MD, along with Drs. Olsen and Barrett and others who do ray tracing, realized that the different lens shapes now being used by different companies made a difference, so they added a lens-shape constant to their equations. This addition gave us another 2 or 3 percent improvement. Now in most large studies, about 80 percent of eyes are within 0.5 D of target. Incorporating tomographic data would increase our overall accuracy another percent or two, but we’d still have a significant number of patients with a half diopter or more of residual refractive error.” —CK |
“With that in mind, I worked with Giacomo Savini, MD, and Leonardo Taroni, MD, from Bologna, Italy, to create a new version of the formula called the Hoffer QST,” he says. “We updated the algorithms, added AI, and added the requirement of inputting gender and preop anterior chamber depth as part of the data. We’ve now done studies with a large series of Asian and Caucasian eyes, and the results indicate that the updated formula is equal to or better than the other current formulas, depending on which parameter you’re comparing and which subgroup of patients you focus on. In any case, results with the Hoffer QST are within the same accuracy range as the other current formulas.4 Statistically, there’s no significant difference between them.
“So now, I tell doctors who may still be using the Hoffer Q formula to please upgrade to the Hoffer QST,” he says. “The QST will soon be available in the new REVO FC biometer, from Optopol, including the toric IOL calculator developed by Dr. Savini and Kristian Naeser, MD, which is quite sophisticated.”
Dr. Hill also says his development team recently released an updated version of the Hill-RBF calculation method, which employs artificial intelligence. “The new version is more accurate than previous versions, as was demonstrated in a study published earlier this year,” he says.5 “That study showed that Hill-RBF version 3.0 has a ±0.5 D rate equal to or better than current IOL power calculation methods—although there’s no question that the Barrett Universal II also produces excellent outcomes.
“All versions of the RBF method were created in tandem with the engineers and mathematicians at MathWorks, which employs some of the most sophisticated artificial intelligence experts in the world,” he adds. “I also work regularly with Jonas Haehnle, PhD, the Haag-Streit mathematician in Switzerland. (Dr. Hill recently summarized the current status of IOL power selection using artificial intelligence in the Richard Lindstrom Lecture at the 2022 meeting of the American Society of Cataract and Refractive surgery in Washington, DC.)
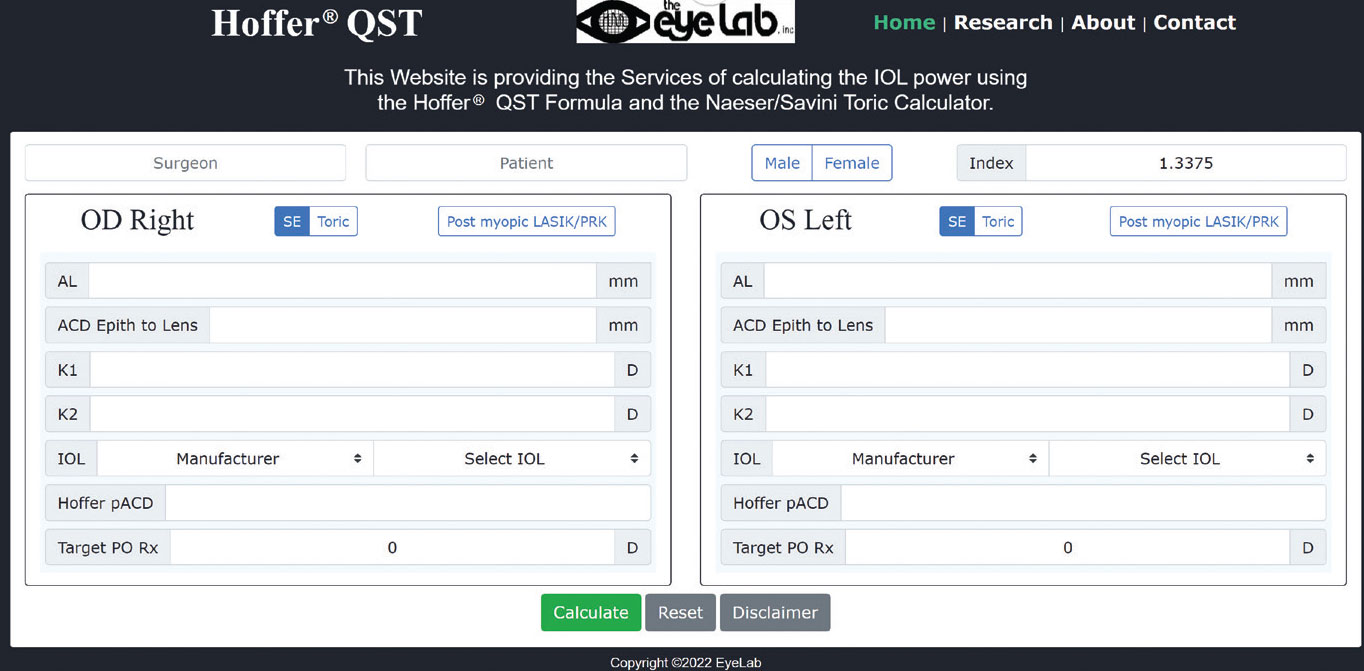
Another aspect of offering cutting-edge formulas today is giving surgeons easy access to them online (since a limited number are currently built into the popular biometers). Dr. Hoffer says that in December of 2020 his team created a website for his new Hoffer QST formula, at HofferQST.com. “We included the Naeser-Savini toric IOL calculator, as well as a post-LASIK version of the formula,” he says.
“However, we decided to offer additional features,” he says. “Our website will calculate your personalized lens factor [pACD] for using with the new formula. We’ve also added a feature for researchers looking to compare formulas. The website includes another spreadsheet that allows you to enter your data, and it calculates the median absolute error, percentage of eyes within a quarter-diopter of target, and so forth, using different formulas.”
Maximizing Your Biometry
Of course, a good formula is worthless if your measurements aren’t accurate. In addition to Zeiss’s IOLMaster 700 and Haag-Streit’s LenStar LS 900, a variety of high-tech biometers are currently available, including the Aladdin from Topcon EU; Nidek’s AL-Scan; Zeimer’s Galilei G6; Tomey’s OA-2000; the Pentacam AXL from Oculus; and Alcon’s Argos. However, the technology can’t provide accurate measurements without assistance from the user. Surgeons offer these strategies to ensure accurate measurements:
• Optimize the ocular surface before doing biometry. “Keratometry has the potential to be one of the least accurate parts of the measurement process,” notes Dr. Hill. “Adi Abulafia, MD, in Israel has shown that if you take a healthy volunteer in their 30s or 40s and do keratometry using a biometer, then bring them back in two days and do keratometry again, you’re likely to get somewhat different numbers. This is due to variations in the ocular surface. In addition, as we get older, the ocular surface becomes less stable.
“To compensate for this, we have our patients do warm compresses twice a day for two weeks before biometry,” he says. “That helps to expel the lipid-rich contents of the meibomian glands, very useful for stabilizing the ocular surface. We also ask the patient to do lid scrubs twice daily to remove any debris that may result from this. Finally, we have the patient use artificial tears frequently—as often as six times a day. All of this improves the ocular surface before biometry.
“When we optimize the ocular surface, the measurement-to-measurement variations we frequently see go away,” he concludes. “If we do topography, the multiple small flat and steep islands that are seen with an unstable cornea disappear. In addition, mildly abnormal aberration profiles tend to normalize. The result is that we end up with more trustworthy measurements.”
 |
|
The new Hoffer QST formula website includes the Naeser-Savini toric IOL calculator and a post-LASIK version of the formula. It will also calculate your personalized lens factor for use with the new formula, and can compare outcomes with different formulas. |
• Do a preoperative screening. Jack T. Holladay, MD, MSEE, FACS, the developer of the Holladay 1, 2 and Refractive Formulas, and president of Holladay Consulting, the distributor of the Holladay IOL Consultant software (hicsoap.com), has a protocol he uses to identify eyes that are likely to produce problematic outcomes. “This approach involves looking at three binocular measures and two monocular measures preoperatively,” he explains. “They allow me to identify the 20 percent of eyes that will fall out of bounds, before I get started.
“In terms of binocular measurements, the first screening criteria is that the predicted IOL powers in a normal patient whose vision is roughly symmetrical in both eyes—which includes 99 percent of patients—should never show more than a 1-D difference,” he says. “The second red flag is mean axial lengths in the two eyes being more than 0.1 mm different. The third warning sign is keratometry of the two eyes showing more than a 0.5-D difference. These findings almost never appear in a normal patient, so any of them is a red flag. If you find them, you should repeat that patient’s measurements.
“In addition, there are two monocular criteria,” he says. “First, the keratometer on every optical biometer has a standard deviation measurement. Doctors rarely look at it, because it’s usually zero—but not always. It’s worth checking, because that standard deviation should never be more than 30 µm, which equals about 0.2 D. If the standard deviation is greater than that, that’s a red flag that this patient has an abnormal cornea. It’s telling you that’s these measurements aren’t good and corneal measurements should be taken on a topographer or tomographer.
“The second monocular criterion is that the signal-to-noise ratio for your axial length measurements should be more than 10, 15 or 20,” he says. “A ratio lower than that means the axial length measurement isn’t reliable. In that case you should walk the patient over to the ultrasound and get another measurement.
“Any one of these red flags indicates that the patient has a higher probability of having a refractive surprise,” he concludes. “I tell doctors to tape a sheet of paper listing these points to the optical biometer. That list will remind your technician to check for these red flags. If the patient fails any one of those five tests, walk the patient to the topographer or ultrasound machine, or at least repeat the measurements and spend a little more time on the patient.”
Dr. Holladay notes that this protocol isn’t a cure-all. “Even if you do this, you won’t get 100 percent of your patients within a half diopter of target,” he says. (See sidebar, above.) “This screening list is helpful, however, because the surgeons who don’t check for these red flags are probably only getting 70 percent of patients within 0.5 D. Taping this list to your biometer will start improving your results on day one.”
• Do the work required to learn about your tools. “The surgeon is the one who needs to understand the measurement tools at their disposal, inside out,” says Dr. Hill. “The doctor is the one who should give guidance and instructions to the staff. As the technology changes, we as physicians should take the time to learn about the new developments; that’s part of our job.”
Frequently Asked Questions
Many surgeons still inquire about these issues:
• What’s the best way to improve outcomes? “The only way to know the accuracy of this exercise is to track refractive outcomes closely and take whatever steps may be necessary to improve your accuracy,” says Dr. Hill. “Unfortunately, most surgeons rarely do this.”
• What level of accuracy should I be aiming for? Dr. Hill says that some surgeons have enlisted his help to compute their half-diopter accuracy. “I find the ±0.5 D accuracy of most surgeon datasets to be between 78 and 80 percent,” he says. “That’s what surgeons typically discover when they look objectively at their refractive outcomes for the first time. In the beginning, improving outcomes is a slow uphill climb, and every increase in accuracy is hard-won, involving sequential changes.
“In my experience, if you do everything correctly, your ±0.5 D accuracy can get as high as 90 percent,” he continues. “If you closely follow biometer validation criteria, optimize the ocular surface before biometry and use the best formulas, this level of accuracy is achievable. If your ±0.50 D accuracy for normal eyes is less than that, there’s room for improvement.”
• Should we take multiple measurements? “It’s generally best to use a single set of accurate measurements rather than routinely obtain multiple measurements,” says Dr. Hill. “The idea that we should take measurements from multiple devices and use multiple formulas is from another time. I recommend using the best of what we have. If you’re using a biometer, this can be accomplished by carefully applying validation criteria to each group of measurements.”
Can we reach 100 percent? “The unfortunate reality is, we can’t get 100 percent of patients where we want them with the technology we have today,” Jack T. Holladay, MD, MSEE, FACS, the developer of the Holladay 1, 2 and Refractive Formulas, and president of Holladay Consulting, explains. “We’ve reached a ceiling because we’re limited by three things. First, the accuracy of our measurements has limits, and slight inaccuracies in multiple measurements can add up. Second, we’re measuring a living, changing system, and every patient’s cornea is unique. Third, there’s a limit to our ability to predict where the IOL will sit inside the eye.” What about the addition of artificial intelligence to the formulas? “Artificial intelligence can’t compensate for the limited accuracy of our measurements,” he notes. “The point is that when it comes to not getting every patient to the target, the formula isn’t really the problem,” he says. “It can’t improve the precision of our measurements or tell us where the IOL will end up sitting. The reason we get as many within 0.5 D as we do is that the small errors often push the outcome in opposite directions, nullifying each other. But in some cases, the small errors push the numbers in the same direction, causing the patient to end up outside the half-diopter range.” Dr. Holladay adds that the one ray of hope he sees is RxSight’s Light Adjustable Lens. “After surgery, that lens can be fine-tuned to be extremely close to the target,” he points out. “The number of happy patients can thus go up to 99 percent.” —CK |
• Should we still use different formulas for different axial lengths? Experts agree that current formulas do well regardless of axial length. “In our practice,” Dr. Hill notes, “we only look at two calculation methods: Graham Barrett’s Universal II formula and version 3 of Hill-RBF, the one I developed.”
• What about eyes with prior refractive surgery? For these eyes, Dr. Hill recommends using the ASCRS post-refractive online calculator, created with Li Wang, MD, Ph.D. and Douglas Koch, MD, at iolcalc.ascrs.org. “Many different measurement devices can be used with these eyes, but the Lenstar, the IOLMaster and the Zeiss Atlas topographer have the greatest overall utility,” he says. “We do this type of calculation every day and get the best results using the Barrett True K method.”
• Is it worth investing in the latest equipment? “Some surgeons and their staff believe that they can get better results by throwing more money at the process,” Dr. Hill notes. “However, a tool is only as good as the person using it. The most important tool we all have is between our ears. I often take the time to do some of the measurements with my staff. We learn from each other.”
• Is one calculation method superior to all others? Dr. Hill notes that comparing the accuracy of various calculation methods can be tricky. “One reason there’s so much confusion about whether one formula is more accurate than another is that researchers often use comparative methods that are statistically inappropriate,” he says. “When comparing things that produce widely variable outcomes—and IOL power calculation is the poster child for this kind of comparison—then using standard statistical tools becomes meaningless. To make such a comparison meaningful requires a heteroscedastic statistical method [designed to compensate for that variability].” (He points out that the comparisons done in the recent study that tested the Hill-RBF 3.0 formula were done using a heteroscedastic method.)
All the Answers in One Place
“Most of the new formulas aren’t available on a biometer,” notes Dr. Hoffer. “That means the doctor has to take the data that he gets from the biometer, go to the website and have someone put in the data and get a printout. It takes a fair amount of time and effort to do this for more than one formula, if you want to see how their outcomes compare.
“However, about a year and a half ago, a young ophthalmologist in Buenos Aires, Argentina—Dante Buosanti, MD—wrote to me and asked if I’d allow him to put the Hoffer QST formula on his new website,” he says. “There’s a term called ‘web scraping,’ which refers to gathering data from another website and bringing it back. His idea was to allow surgeons to access multiple formulas at one location.
“You’d go to the website, click boxes for all of the formulas you’d like to use and enter all of your data,” he continues. “If you were missing something that a particular formula requires, such as patient gender, a note would pop up. Once you entered the data—which you only have to do once—you’d press the ‘calculate’ button, and it would send the data to each website, get the calculations done and bring the results back to this website.
“I thought Dr. Buosanti’s idea was terrific, so I gave him permission to include the Hoffer QST,” Dr. Hoffer says. “He’d already gotten permission to include the RBF 3.0 and the Ladas Super formula.
“Actually, he doesn’t need the approval of the website owners to do this,” Dr. Hoffer notes. “If you create a public website, anybody can go to it and enter any data they want to and get an answer. But I recommended that he get everyone’s approval—after all, their names are on the formulas. He said that was his plan. Initially, several formula authors were reluctant to give their permission, but because I know most of them, I pursued the matter.
“Finally, I suggested that instead of Dr. Buosanti doing this by himself, his website should be posted under the auspices of an organization,” Dr. Hoffer continues. “I contacted the president of ESCRS and asked whether they’d be interested in having this on the ESCRS website, which currently doesn’t offer a calculator. (ASCRS already has a calculator on its website, and was not interested in expanding.) He took the suggestion to the Board, and they liked the idea enough to set up a committee to work on it.
“The upshot,” Dr. Hoffer says, “is that in September of this year, ESCRS will add this to their website for the whole world to use, with the approval of every formula creator. Dr. Kane is on board, and others have agreed to add theirs after the website goes live.
“To me, this is earth-shaking,” Dr. Hoffer concludes. “The process of calculating lens power is going to change. You won’t have your biometer do it; you’ll go to the ESCRS website and get a printout with all the different results you’d like to see, on one page. It’s a cooperative effort, and it should have a big impact. I think it will make IOL calculation easier for people and improve results worldwide. I’m excited to see this happening!”
Dr. Holladay is a consultant for Carl Zeiss, M&S Technologies, Oculus, Sonomed, Acutome, Visia Imaging, Zeimer Ophthalmics, Heidelberg Engineering, Medisoft Imaging and Ellex. Dr. Hill is the author of the Hill-RBF artificial intelligence IOL power calculation method and a Haag-Streit AG, Switzerland consultant. Dr. Hoffer receives royalties for the use of his formulas in commercially available biometers.
1. Debellemanière G, Dubois M, Gauvin M, et al. The PEARL-DGS Formula: The development of an open-source machine learning-based thick IOL calculation formula. Ophthalmology 2021;232:58-69.
2. Kane JX, Van Herdeen A, Atik A, Petsoglou C. Intraocular lens power formula accuracy: Comparison of 7 formulas. J Cataract Refract Surg 2016;42:1490-1500.
3. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmol 2018;125:169-178.
4. Taroni L, Hoffer KJ, Pellegrini M, Enrico L, Schiavi C, Savini G. Comparison of the new Hoffer QST with four modern accurate formulas. J Cataract Refract Surg 2022, in press.
5. Tsessler M, Cohen S, Wang L, Koch DD, Zadok D, Abulafia AJ. Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using a heteroscedastic statistical method. Cataract Refract Surg 2022;48:1:37-43.