HC-HA/PTX3 Complex in AM
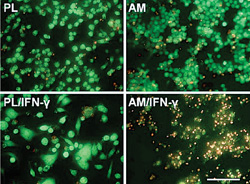
In 2006, we first reported that AM stromal matrix exerts an anti-inflammatory action by inducing apoptosis of IFN-γ–activated monocyte/macrophage RAW264.7 cells (See Figure 1) and that such an action is not caused by nitric oxide but instead by the downregulation of anti-apoptotic NF-κB and Akt-FKHR signaling pathways.1 Subsequently in 2008, we demonstrated that such an anti-inflammatory action is retained in soluble AM extract (AME). AME upregulates IL-10, downregulates TNF-α and IL-6, and suppresses the activation of RAW264.7 cells by IFN-γ, LPS and IFN-γ/LPS.2 These findings suggest the key molecule(s) in AM responsible for its anti-inflammatory and other biological actions can be extracted and possibly be identified and isolated.
Indeed, in 2009, we successfully purified a complex designated as “HC-HA/PTX3” from AME.3 HC-HA/PTX3, first found in cumulus-oocyte complex (COC) surrounding the ovulated oocyte, is vital for subsequent fertilization.4,5 It is formed by tight association between pentraxin 3 (PTX3) and HC-HA, which consists of high molecular weight hyaluronic acid covalently linked to heavy chain 1 (HC1) of inter-α-trypsin inhibitor. IαI is mainly secreted by the liver and present in the blood at considerably high concentrations (0.15 to 0.5 mg/ml).6 It is composed of a common light chain and two heavy chains (HC1 and HC2). The light chain is a typical proteoglycan molecule having a single chondroitin four-sulfate chain.
|
Retains Anti-inflammatory Action
Our studies have also shown that HC-HA/PTX3 purified from AM retains AM’s anti-inflammatory action by exerting the following effects:
1) HC-HA/PTX3 promotes apoptosis of pro-inflammatory but not resting neutrophils and macrophages. During inflammation, neutrophils are among the first recruited to engulf pathogens and damaged tissues before their eventual apoptosis.18-20 Pathologically, delayed neutrophil apoptosis leads to a prolonged inflammation, which is a hallmark of many inflammatory diseases.21,22 We have reported that apoptosis of freshly-isolated neutrophils activated by fMLP or LPS is promoted only when they are treated by soluble HC-HA/PTX3, but not HA or PBS control.23 We also report that soluble HC-HA/PTX3, the same as AM and AME, dose-dependently promotes apoptosis of RAW264.7 cells activated by IFN-γ, LPS, or IFN-γ/LPS.2,3,23
2) HC-HA/PTX3 enhances phagocytosis of apoptotic neutrophils by macrophages. Clearance of apoptotic neutrophils by macrophages is essential for inflammation resolution.24-26 We have reported that soluble HC-HA/PTX3, but not HA, is effective in enhancing phagocytosis of apoptotic neutrophils by resting macrophages (about sevenfold vs. PBS control). Meanwhile, immobilized HC-HA/PTX3 is more potent in promoting phagocytosis of apoptotic neutrophils by LPS-activated macrophages (about 2.5-fold).23
 |
4) HC-HA/PTX3 suppresses activation of CD4+ T cells. Naïve CD4+ T cells can be activated to proliferate and differentiate into Th1, Th2, Th17 or Tregs.34-36 Th1 cells secrete IFN-γ and IL-2 to enhance pro-inflammatory responses.37,38 This action can be downregulated by Tregs that is activated by M2 macrophages.39 Our study shows the soluble HC-HA/PTX3 suppresses the proliferation and production of IFN-γ and IL-2 and expression of T cell activation markers (CD25 and CD69) while significantly promoting the expansion of CD25+/FOXP3+ T cells.31 These data strongly suggest that HC-HA/PTX3 suppresses activation of CD4+ T cells into Th1 cells.
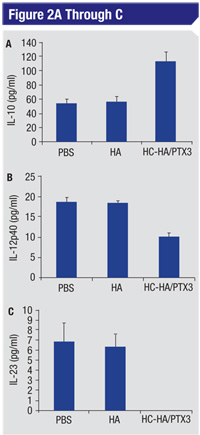
5) HC-HA/PTX3 suppresses the macrophage influx to LPS-elicited corneas and prolongs survival of corneal allografts. While intrastromal injection of LPS elicited notable influx of EGFP+ macrophages to the corneal periphery in Mafia mice from day one to day five,40,41 subconjunctival injection of HC-HA/PTX3 significantly suppresses the infiltrated macrophages. Further, the infiltrated macrophages are polarized towards an M2 phenotype by expressing higher M2 markers (Arg-1 and IL-10) but lower M1 markers (IL-12p35 and IL-12p40).31 Using a murine orthotropic corneal transplantation model,42-45 we have found that subconjunctival injection of HC-HA/PTX3 prolongs the significant survival of allografts compared to PBS injection.31 These data suggest that HC-HA/PTX3 may be used to exert a potent anti-inflammatory action leading to suppression of (alloreactive) immune activation in vivo.
HC-HA/PTX3, a unique matrix component purified from human AM, retains AM’s multifactorial anti-inflammatory actions. It is foreseeable that HC-HA/PTX3 can be used as a new class of biologics to treat ocular inflammatory diseases (e.g., proliferative diabetic retinopathy, trauma and subretinal fibrosis secondary to age-related macular degeneration and other choroidal neovascular processes) as well as similar pathological processes in other parts of the body. REVIEW
Dr. Tseng is a physician scientist and the chief scientific officer of TissueTech Inc, where both Dr. He and Dr. Zhang serve as research scientists. Contact Dr. Tseng at Ocular Surface Center, 7000 SW 97 Ave., Ste. 213, Miami, Fla. 33173. Phone: (305) 274-1299; fax: (305) 274-1297; e-mail: stseng@ocularsurface.com.
Supported in part by grants from National Institute of Health, National Eye Institute. Additional support is from a research grant from TissueTech Inc. and an unrestricted grant from Ocular Surface Research & Education Foundation, Miami.
1. Li W, He H, Kawakita T, Espana EM, Tseng SCG. Amniotic membrane induces apoptosis of interferon-gamma activited macrophages in vitro. Exp Eye Res 2006;82:282-292.
2. He H, Li W, Chen SY, et al. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci 2008;49:4468-4475.
3. He H, Li W, Tseng DY, et al. Biochemical Characterization and Function of Complexes Formed by Hyaluronan and the Heavy Chains of Inter-α-inhibitor (HC•HA) Purified from Extracts of Human Amniotic Membrane. J Biol Chem 2009;284:20136-20146.
4. Zhuo L, Yoneda M, Zhao M, et al. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem 2001;276:7693-7696.
5. Salustri A, Garlanda C, Hirsch E, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004;131:1577-1586.
6. Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem 2004;279:38079-38082.
7. Salier J-P, Rouet P, Raguenez G, Daveau M. The inter-α-inhibitor family: from structure to regulation. Biochem J 1996;315:1-9.
8. Zhang S, He H, Day AJ, Tseng SC. Constitutive expression of inter-α-inhibitor (IalphaI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J Biol Chem 2012;287:12433-12444.
9. Lee TH, Wisniewski H-G, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding protein, closely related to the adhesion receptor CD44. J Cell Biol 1992;116:545-557.
10. Wisniewski H-G, Maier R, Lotz M, Klampfer L, Lee TH, Vilcek J. TSG-6: a TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J Immunol 1993;151:6593-6601.
11. Wisniewski HG, Vilcek J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev 1997;8:143-156.
12. Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol 2008;28:1-13.
13. Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu Rev Immunol 2010;28:157-183.
14. Inforzato A, Rivieccio V, Morreale AP, et al. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J Biol Chem 2008;283:10147-10161.
15. Inforzato A, Jaillon S, Moalli F, et al. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens 2011;77:271-282.
16. Scarchilli L, Camaioni A, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: Implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem 2007;282:30161-30170.
17. Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive Expression of PTX3 by Human Amniotic Membrane Cells Leads to Formation of the HC-HA/PTX3 Complex. J Biol Chem. 2014.
18. Frasch SC, Nick JA, Fadok VA, Bratton DL, Worthen GS, Henson PM. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem 1998;273:8389-8397.
19. Pongracz J, Webb P, Wang K, Deacon E, et al. Spontaneous neutrophil apoptosis involves caspase 3-mediated activation of protein kinase C-delta. J Biol Chem 1999;274:37329-37334.
20. Khwaja A, Tatton L. Caspase-mediated proteolysis and activation of protein kinase Cdelta plays a central role in neutrophil apoptosis. Blood 1999;94:291-301.
21. Lutgens E, Lievens D, Beckers L, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med 2010;207:391-404.
22. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: Subsets and functions. Nat Rev Cardiol 2010;7:77-86.
23. He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized Heavy Chain-Hyaluronic Acid Polarizes Lipopolysaccharide-activated Macrophages toward M2 Phenotype. J Biol Chem 2013;288:25792-25803.
24. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 1999;17:593-623.
25. Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol 2012;12:492-502.
26. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723-737.
27. Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 2011;12:231-238.
28. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-969.
29. Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity 2010;32:593-604.
30. Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest 2012;122:787-795.
31. He H, Tan Y, Duffort S, Perez VL, Tseng SC. In Vivo Downregulation of Innate and Adaptive Immune Responses in Corneal Allograft Rejection by HC-HA/PTX3 Complex Purified from Amniotic Membrane. Invest Ophthalmol Vis Sci 2014 Mar 19;55(3):1647-56.
32. Croxford AL, Mair F, Becher B. IL-23: One cytokine in control of autoimmunity. Eur J Immunol 2012;42:2263-2273.
33. Chen X, Zhao S, Tang X, Ge H, Liu P. Neutralization of mouse interleukin-17 bioactivity inhibits corneal allograft rejection. Mol Vis 2011;17:2148-2156.
34. Ayliffe W, Alam Y, Bell EB, McLeod D, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol 1992;76:602-606.
35. Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res 2007;32:1005-1016.
36. Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci 1999;40:2614-2621.
37. Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell 1994;76:241-251.
38. Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179-188.
39. Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237-246.
40. Carlson EC, Drazba J, Yang X, Perez VL. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47:241-248.
41. Chinnery HR, Carlson EC, Sun Y, et al. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation. J Immunol 2009;182:2738-2744.
42. Streilein JW. New thoughts on the immunology of corneal transplantation. Eye 2003;17:943-948.
43. Amescua G, Collings F, Sidani A, et al. Effect of CXCL-1/KC production in high risk vascularized corneal allografts on T cell recruitment and graft rejection. Transplantation 2008;85:615-625.
44. Tan Y, Cruz-Guilloty F, Medina-Mendez CA, et al. Immunological disruption of antiangiogenic signals by recruited allospecific T cells leads to corneal allograft rejection. J Immunol 2012;188:5962-5969.
45. Tan Y, Abdulreda MH, Cruz-Guilloty F, et al. Role of T cell recruitment and chemokine-regulated intra-graft T cell motility patterns in corneal allograft rejection. Am J Transplant 2013;13:1461-1473.