When I was a first-year resident, I thought that angle-closure glaucoma was one of the easier topics to master: Perform an iridotomy and the problem is solved. But as my training progressed, I realized there’s a lot more to it. Even with a patent iridotomy and glaucoma medications, patients often end up being surgical candidates because of the development of chronic angle closure.1 Today, I encourage trainees to manage angle-closure glaucoma patients with a longitudinal approach, by performing serial gonioscopy and following eye pressures closely. It’s not “one and done” with these patients. They can develop significant problems later.
The challenge and reward in treat-ing angle-closure glaucoma is that managing these patients involves clinical acumen; in many ways it’s very esoteric. You can determine the nature and mechanism of a patient’s disease, and then use that knowledge to tailor your surgical choices—an approach that has served me and my patients well.
Here, I’d like to discuss the different treatment approaches, both new and traditional, and offer my perspective on the pros and cons of each.
The Gold Standards
In terms of tried-and-true options for managing angle-closure glaucoma (beyond iridotomy and iridectomy), two options stand out:
• Trabeculectomy. Today, trabeculectomy remains the most commonly performed surgery for chronic angle-closure glaucoma. It’s an effective approach with a greater than 70-percent success rate and aver-age pressure lowering of about 30 percent.2 However, trabeculectomy is falling out of favor with some surgeons because of its associated complications. Up to 50 percent of patients with angle-closure glaucoma who receive a trabeculectomy end up needing additional surgery, and more than 30 percent develop a cataract, at least in the American cohort of angle-closure patients.3 Furthermore, there’s always the feared complication of malignant glaucoma. Known risk factors for this include the presence of the crystalline lens, a higher pre-operative IOP and the absence of an iridotomy.2
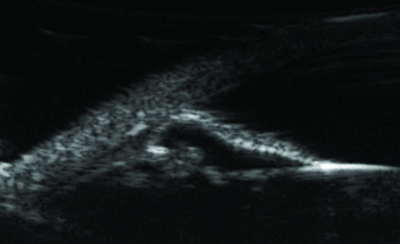 |
| Patients with angle closure often end up as surgical candidates because despite a patent iridotomy, some continue to develop chronic angle-closure glaucoma. |
In regards to the latter group, an angle-closure glaucoma patient might not receive an iridotomy for a number of reasons. If a patient presents with a lot of peripheral anterior synechiae and high pressure, there’s a risk that an iridotomy will cause enough inflammation to zip up the angle, possibly 360 degrees. For that reason some doctors will forgo an iridotomy and proceed straight to performing a trabeculectomy. And while most surgeons choosing to perform a trabeculectomy would automatically also perform a surgical iridectomy, a small percentage of surgeons have modified their technique for trabeculectomy and forgo the iridectomy.
I’m a firm believer that it’s important to create an iridectomy—especially in chronic angle-closure glaucoma patients—during trabeculectomy surgery. In addition, I make sure it’s a large iridectomy to avoid any question about patency. I recently performed a trabeculectomy on a patient who had an existing laser iridotomy, but I still performed a surgical iridectomy during the trabeculectomy to mitigate the risk of aqueous misdirection.
• Tube shunts. For those chronic angle-closure glaucoma patients at high risk of failure with a trabeculectomy, tube shunts can also be effective. Surgeons may be especially likely to opt for a tube shunt when prior glaucoma surgery has failed in cases of secondary angle-closure glaucoma, such as neovascular glaucoma, traumatic glaucoma, ICE syndrome and developmental glaucomas.4
However, angle-closure glaucoma patients are still prone to tube-re-lated complications. In particular, patients with angle closure often have very shallow anterior chambers, causing the tube to end up closer to the cornea, potentially leading to corneal decompensation. Also, some of these patients, such as those with neovascular glaucoma, may not have an iridotomy, putting them at risk of malignant glaucoma. There’s also a risk of tube occlusion with proximity to the iris.
 |
| More overt pathologies of the iris in angle closure include peripheral anterior synechiae (above) and iris neovascularization. |
Beyond the possibility of com-plications, the biggest problem with these gold-standard approaches to managing angle-closure glaucoma is that they don’t address the underlying cause of the problem. Underlying causes can include lens vault and position; ciliary body anatomy, including anterior rotation, cysts and tumors; iris physiology, including peripheral anterior synechiae; and trabecular meshwork dysfunction, sometimes referred to as “mixed mechanism glaucoma.”5,6 For that reason, surgeons are now starting to consider alternative surgical techniques that might address the underlying mechanisms more directly. These options might not be advisable in every situation, but if you’re man-aging an angle-closure glaucoma patient who is stable or in the early stages of disease, I think addressing the underlying mechanism is a good place to start.
Option 1: Cataract Surgery
Because lens vault and position is a possible mechanism of angle-closure glaucoma, removing the lens makes sense as a possible treatment. In years past, performing cataract surgery alone wasn’t considered a first-line treatment for angle-closure glaucoma, but today’s small-incision cataract surgery is considerably safer for the patient. In addition, thanks in part to the EAGLE trial,7 lens extraction has been more widely accepted as a treatment option in these patients. The EAGLE trial looked at clear lens extraction vs. laser iridotomy for angle-closure glaucoma; the majority of the participating patients had high pressures. In the patients with high pressures, both quality of life and other IOP outcome measures were favorable after clear lens extraction.
A review looking at the effect of phacoemulsification cataract surgery alone on eye pressure in primary angle-closure glaucoma patients8 found that cataract surgery alone reduced the pressure by an average of 30 percent, and medication requirements dropped by half. So there’s plenty of evidence that in the right group of angle-closure glaucoma patients, cataract surgery alone is a reasonable option.
Because of this evidence, I’ve offered clear lens extraction to some of my mild angle-closure glaucoma patients. I believe this makes the most sense in patients who are already of presbyopic age, with elevated pressure and occludable angles—especially with thick, brown irides. In those patients an iridotomy could incite more inflammation and possibly even cause peripheral anterior synechiae, which could end up making the prob-lem worse.
Incidentally, the biggest barrier I’ve encountered with patients in this situation is the perception that undergoing surgery is riskier than treatment via laser. As specialists, we’re aware that an iridotomy can produce inflammation and require considerable monitoring to avoid pressure spikes that can be difficult to manage—but that’s not what a patient might assume. So, it’s helpful to have clinical studies such as the EAGLE trial to support our recommendation when we’re discussing this with patients.
Option 2: Treat the Ciliary Body
In many of these patients, it’s clear that the angle-closure problem is associated with ciliary body anatomy, which may have some anterior rotation. Some surgeons refer to this as plateau iris configuration or syndrome. (Of course, this configuration can be subtle and the anatomy in angle-closure glaucoma falls along a spectrum.) For example, sometimes you’ll see a large crystalline lens and a slight anterior rotation of the ciliary body. Such a patient might not be thought of as having plateau iris syndrome, but the ciliary body is still contributing to the appositional closure of the angle.
One approach to altering the position of the ciliary body is endoscopic cyclophotocoagulation, or ECP.9 Furthermore, if someone is at high risk of having trouble with intraocular surgery, or if an ECP ma-chine is not available, transscleral cyclophotocoagulation can produce a very similar effect.10 In patients at high risk for malignant glaucoma after incisional surgery, this is a good option.
It’s worth noting that ciliary body cysts may sometimes contribute to the anterior rotation of the ciliary body, complicating the treatment. In those cases, ultrasound biomicroscopy can be used to help establish the anatomy. If ECP is available, the endoscope can also allow you to directly visualize and shrink the anterior ciliary processes that are contributing to the narrow angle anatomy.
Generally, I only attempt to treat ciliary cysts if they’re diffuse or large. Unfortunately, cysts can recur in some cases. That’s another reason to do this in combination with cataract surgery; by removing the bulk of the lens you’re also opening up the angle, increasing the odds of a good outcome.
Option 3: Goniosynechialysis
Iris physiology, including iris volume, thickness and dynamic changes during constriction and dilation, have also been implicated as possible underlying causes of angle-closure glaucoma.11 In that category, peripheral anterior synechiae can develop with long-standing appositional angle closure. Studies have found that goniosynechialysis can be helpful in treating angle closure, especially in combination with cataract surgery.12,13 (Note: This may not be true in extreme cases of neovascular glaucoma or ICE syndrome.)
| If your angle-closure patient is stable or has mild disease, and you’re doing regular gonioscopy, I think addressing the underlying mechanism is a good place to start. |
This is most likely to be effective in patients who’ve had a shorter duration of angle closure. For ex-ample, if a patient has had an acute episode of angle-closure glaucoma, you might do goniosynechialysis and cataract surgery once the eye is quiet. Goniosynechialysis is less likely to be helpful when the angle closure and PAS have been present for a longer time (more than six months or so), because in that situation, this procedure tends to cause bleeding and the PAS tend to re-form later. For that reason it’s helpful to know the history of the patient.
This is one reason that I’m a big proponent of performing gonioscopy, which has become something of a lost art. In fact, when treating angle-closure glaucoma patients, I sometimes perform gonioscopy every six months, because I find that it re-veals a lot of really useful information.
An Additional Concern …
When a patient has a narrow angle, it’s easy to focus on the problems associated with that condition to the exclusion of other possible issues. In fact, the trabecular meshwork is often a contributing factor in narrow-angle glaucoma.
I’ve seen many patients with appositional closure who’ve had an iridotomy or cataract surgery that successfully opened up the angle. Afterwards, you can see the trabecular meshwork beautifully, with no PAS—yet the patient continues to have high pressures. Presumably that’s because the trabecular meshwork has also become dysfunctional. In fact, it’s been shown that acute and chronic angle-closure patients often have pathology in the trabecular meshwork.14
I think it’s very reasonable to treat those patients as you’d treat high-tension glaucoma patients. They usually respond well to canal-based surgery with or without cataract surgery, sometimes done in combination with goniosynechialysis. Furthermore, studies have shown that selective laser trabeculoplasty can lower the pressure in these primary angle-closure glaucoma patients, at least in the short term.15
I find that in many cases, treating the trabecular meshwork in addition to the narrow angle results in pretty significant pressure lowering. Unfortunately, there haven’t been any large-scale studies on this yet, so at this point the evidence is mostly anecdotal.
Proceed with Caution
The idea of trying to treat the underlying cause of the angle closure sounds like a reasonable approach, raising the question: Why not simply start treating every angle-closure glaucoma patient with something other than a trabeculectomy or tube? The answer is that these newer alternatives may not be appropriate in some situations.
For example, I recently treated an African-American patient with chronic angle closure, very thick brown irides and a sizable cataract. Despite an iridotomy, his pressures spiked to 50 mmHg, lowering only to the high 20s with multiple medications. The patient had documented visual-field losses with uncontrolled pressures in the previous eight months. In cases like that, there’s very little margin of error. If cataract surgery alone or an ECP doesn’t work to lower the pressure, the patient could continue to lose vision and risk fixation. In a case like this where you really need to get the pressure down, I’d still opt for a trabeculectomy.
Of course, trying alternative options such as cataract surgery, ECP or goniosynechialysis may be perfectly reasonable for many angle-closure glaucoma patients. Although not advisable in every situation, if your angle-closure glaucoma patient is stable or has mild disease—and you’re doing regular gonioscopy so you know what’s happening in the angle—I think addressing the underlying mechanism is a good place to start. REVIEW
Dr. Turalba is chief of ophthalmology and visual services at Atrius Health and part-time assistant professor at Harvard Medical School. She reports no financial ties to any product discussed in this article.
1. Choi JS, Kim YY. Progression of peripheral anterior synechiae after laser iridotomy. Am J Ophthalmol 2005;140:1125–1127.
2. Romero P, et al. Trabeculectomy with Mitomycin-C: Outcomes and risk factors for failure in primary angle-closure glaucoma. J Glaucoma 2018;27:101–107.
3. Tham C, et al. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology 2013;120:62-67.
4. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012;153:5:789-803.e2.
5. Suwan et al. Qualitative and quantitative evaluation of acute angle-closure mechanisms. BMC Ophthalmology 2017;17:246.
6. Li et al. Differences between fellow eyes of acute and chronic primary angle closure (glaucoma): An ultrasound biomicroscopy quantitative study. PLoS ONE 2018;13:2.
7. Azuara-Blanco, et al. Effectiveless of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomized controlled trial. Lancet 2016;388:10052:1389-1397.
8. Chen, et al. The effect of phacoemulsifaction on intraocular pressure in glaucoma patients: A report by the American Academy of Ophthalmology. Ophthalmology 2015;122:7:1294-307.
9. Francis, et al. Endoscopic cycloplasty (ECP) and lens extraction for treatment of severe plateau iris syndrome. J Glaucoma 2016;25:e128-e133.
10. Lai, et al. Diode laser transsscleral cyclophotocoagulation as primary surgical treatment for medically uncontrolled chronic angle closure glaucoma: long-term clinical outcomes. J Glaucoma 2005;14:2: 114-9.
11. Quigley, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma 2009;18:173-179.
12. Shingleton, et al. Surgical goniosynechialysis for angle-closure glaucoma. Ophthalmology 1990; 97:551-556.
13. Kameda, et al. Long-term efficacy of goniosynechialysis combined with phacoemulsification for primary angle closure. Graefes Arch Clin Exp Ophthalmol 2013;251:825-830.
14. Hamanaka, et al. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma. IOVS 2011;52:8849-8861.
15. Narayanaswamy, et al. Efficacy of selective laser trabeculoplasty in primary angle-closure glaucoma: A randomized clinical trial. JAMA Ophthalmol 2015;133:2:206-12.



