As glaucoma goes, angle recession is likely more common than we realize, but it’s still a relatively rare subtype. As such, it’s hard to gather data on the condition, especially considering that it usually occurs decades after the inciting event, making prospective studies difficult to carry out. In fact, since the 1960s, no more than eight studies have been published in a single year on this topic.
Given the dearth of studies on angle-recession glaucoma, many of which are quite small and decades old, it’s worth reviewing the original literature on which we base our current management approaches. Here, I’ll discuss how I approach these cases and which treatments have the most robust evidence behind them.
Pathophysiology
Angle recession is the traumatic separation of the circular and longitudinal fibers of the ciliary body.1,2 Clinically, it’s noted on gonioscopy as an irregularly widened ciliary body. However, the widening often appears uniformly throughout the entire angle and can look quite symmetric. It’s often only with the evaluation of the fellow eye that the asymmetry becomes apparent. This is why it’s important to compare the angle appearance in both eyes when evaluating for recession.
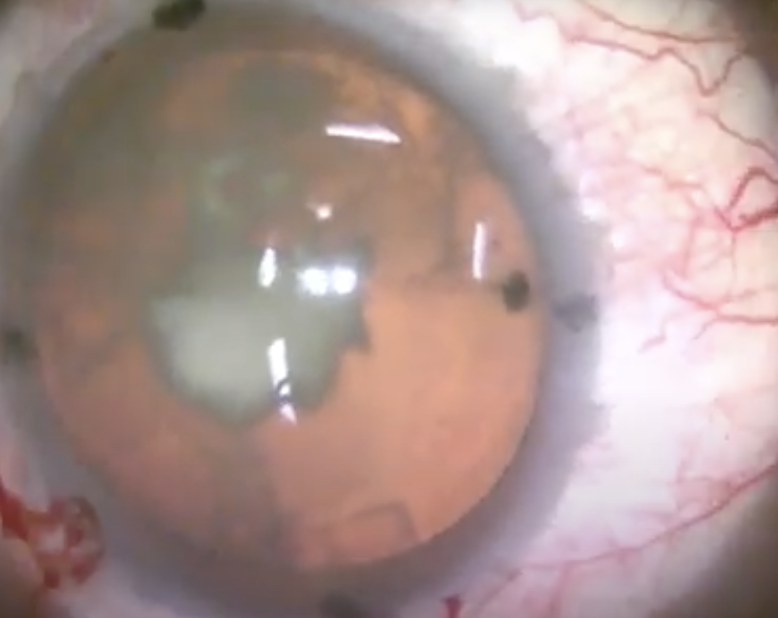 |
| A traumatic cataract with dense central opacity. Blunt ocular trauma may damage a number of structures, including the lens, angle and ciliary body. Delayed onset of angle-recession glaucoma can occur decades after the inciting event. |
Mechanism of Action
Angle recession is the result of non-penetrating or blunt trauma to the eye. With this type of trauma, the force extends anterior to posterior, causing expansion along the equator of the eye, with greatest risk of damage to equatorial structures such as the ciliary body.3
The ciliary body is made up of longitudinal and circular fibers that act in a contradictory fashion. The longitudinal outermost fibers contract anteriorly-posteriorly while the circular innermost fibers contract equatorially. There’s a poorly understood and weaker oblique middle zone between these two areas, and that’s the part at greatest risk of injury. When there’s sudden equatorial expansion, the oblique fibers can be torn. Aqueous humor is also jettisoned toward the ciliary body when there’s sudden change in globe shape, potentially contributing further to injury.
Demographics
After blunt trauma, some individuals will have immediate IOP issues, often due to hyphema or other obstruction of the traditional outflow pathway. But typically, when we think of angle-recession or traumatic glaucoma, we’re looking at the patient population experiencing delayed onset of the disease, usually decades after the inciting event.
A substantial number of patients with a history of trauma have angle recession. Studies have identified angle recession in 71 to 86 percent of traumatized eyes using careful gonioscopy.4 The percentage of eyes with angle recession that go on to develop glaucoma is relatively small at around 10 percent, but this is still a considerable number of patients who often aren’t getting routine eye care because they have no reason to think they’re at risk decades later. The better we can educate patients at the time of trauma, the better chance they’ll have of potentially being seen over the long term to help with risk management.
Risk Factors
Risk factors for glaucoma secondary to angle recession include age and sex (young males are more likely to experience eye trauma), severity of the trauma, and the clinical findings, such as a large amount of angle recession (>240 degrees),5 increased trabecular meshwork pigmentation,6 elevated IOP at presentation7 and hyphema.8-10 In general, the worse the trauma, the higher the risk of secondary glaucoma.
 |
Interestingly, while traumatic glaucoma is unilateral, these patients are more likely to have typical open-angle glaucoma in their fellow eye compared with the normal population. There are a few reasons why this may be, though there’s no definitive answer. One possibility is that these patients are already being monitored and treated for their traumatic glaucoma, and therefore open-angle glaucoma in the fellow eye is identified at a much higher rate than in the standard population.
Another possibility is that patients who develop traumatic glaucoma, particularly decades later, may already be predisposed to open-angle glaucoma in both eyes to begin with, owing to some underlying cause for a compromised outflow system. The trauma may just speed up the disease process and/or increase its severity. These are patients who may have bilateral but still highly asymmetric disease.
Lastly, there may be an autoimmune component to this phenomenon. Trauma to one eye may cause some systemic changes, leading the other eye to develop open-angle glaucoma. However, none of these explanations is fully substantiated, and it’s likely a combination of factors leading to the predisposition.
Clinical Exam
In angle-recession patients, anterior segment findings may include corneal scars, iris trauma such as iridodialysis or traumatic iris defects. The initial trauma may also have caused cataracts or zonular damage leading to a subluxated lens, phacodonesis or pseudophacodonesis.
Gonioscopy of the anterior segment is a key part of the clinical exam for angle recession. The main finding is the widened ciliary body band. Any patient who’s had trauma to the eye should undergo gonioscopy,11 once the eye is stable and any hyphema has resolved. This can help prognosticate future risk for traumatic glaucoma based on the risk factors above. As noted before, it’s important to compare findings with the fellow eye to avoid mistaking uniform widening for normal anatomy.
Routine visual fields and OCT as well as a posterior segment exam for glaucoma should also be performed. On the posterior exam, you may see retinal manifestations of prior trauma, such as chorioretinal lesions.
Unilateral glaucoma is atypical, so it’s important to maintain suspicion for a non-glaucomatous etiology of an optic neuropathy, especially in the absence of elevated intraocular pressure and other compelling factors. Rule out compressive lesions to the optic nerve; infectious, nutritional or autoimmune conditions; and vascular insults to the nerve or within the brain itself.
 |
Management
Intraocular pressure management in patients with angle-recession glaucoma is challenging because their disease can be recalcitrant to more conservative measures. These patients tend to proceed to surgery much more quickly or consistently than the standard open-angle glaucoma patient. Since unilateral vision loss may go unnoticed for years, if the patient has one fully functioning eye, they often aren’t aware of their risk and frequently present late with severely elevated pressures and advanced disease. On the other hand, having a fellow eye with disease can reduce the patient’s overall functional vision loss and impact on activities of daily living.
As mentioned before, as a rarer glaucoma subtype, there are relatively few robust published studies on angle-recession glaucoma. Many of our current management approaches are based on a handful of small, retrospective studies from decades ago.
Is it time to revise some of our management approaches? Here’s what the literature says:
• Medical management. Pilocarpine is relatively contraindicated in angle-recession glaucoma because it’s said to cause a paradoxical rise in intraocular pressure. It’s presumed to decrease uveoscleral outflow, and patients who already have a compromised traditional outflow system could experience a pressure increase.
The data supporting this contraindication comes from a single case report in 1979.12 A single patient was given pilocarpine and their pressure went up. They were then administered a cycloplegic and their pressure came back down. Despite this limited data, I think there’s reason to worry about this effect. There’s likely a good amount of anecdotal information supporting this finding, and certainly, it makes sense mechanistically. The traditional medical management approach for glaucoma is otherwise reasonable for this angle-recession subtype.
• Laser trabeculoplasty. SLT is traditionally contraindicated in traumatic glaucoma, or at least highly discouraged. One of the presumed mechanisms of traumatic glaucoma is an epithelial membrane that forms across the trabecular meshwork, one that isn’t penetrated with traditional laser trabeculoplasty. In fact, treating the trabecular meshwork with ALT was found to produce a blanching effect in some cases, similar to what you’d see with epithelial downgrowth. This was thought to prove that a membrane grows with angle recession and that laser trabeculoplasty doesn’t work well.
Our three major glaucoma textbooks and our BCSC residency educational series all recommend against SLT. This information comes from two retrospective, single-center studies in the early 1980s that looked at a broad range of indications for ALT.13-14 One study included six patients and the other had four patients with angle recession. All did relatively poorly, compared with the broader range of other disease states. However, we rarely use ALT nowadays, and it has a slightly different mechanism of action from SLT.
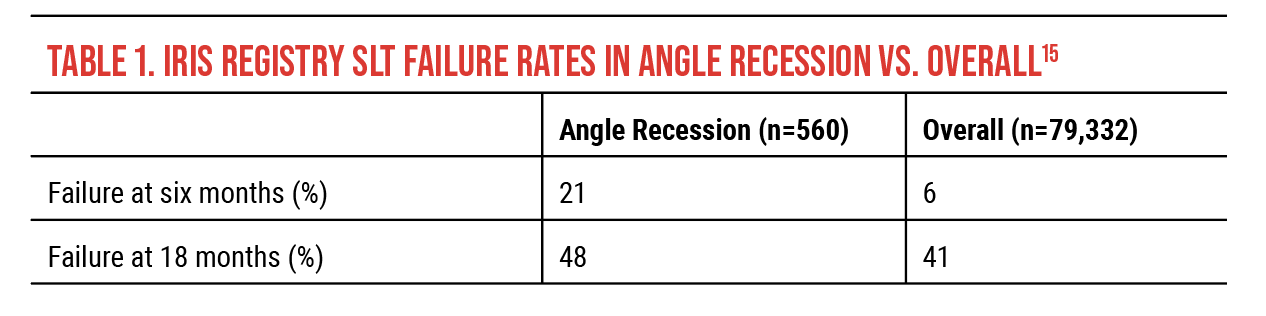
More recently, a much larger 2021 study using IRIS Registry data15-16 looked into factors associated with favorable laser trabeculoplasty outcomes (Table 1). Overall, 79,332 patients had SLT. A total of 560 had angle recession. (Limited conclusions can be drawn since the study didn’t specify the indication for SLT or any patient characteristics.)
For the angle-recession patients who underwent SLT, the study reported a 21-percent failure rate at six months and a 48-percent failure rate at 18 months. While these rates are high, the overall SLT failure rate was 6 percent at six months and 41 percent at 18 months—a mere 7-percent difference between angle recession and all other forms of glaucoma in the study. This difference is statistically significant, but what about the clinical significance? According to this study, there’s a 50-percent chance that SLT will “work” (i.e., avoid surgery). On this basis, I would argue it’s not unreasonable to consider doing SLT in an appropriate patient with angle recession, especially if the next step is surgery.
There certainly is a legitimate concern for the abnormal pathology of the angle compared to other indications for SLT. Laser may not be as effective in these patients. However, we simply don’t have sufficient data to demonstrate whether or not that’s truly the case, and angle recession probably can’t be reduced to a single mechanism. We’re limited diagnostically in terms of identifying what specific angle malfunction a patient has or whether or not an individual patient would respond well or poorly to SLT.
• Trabs and tubes. Angle-recession patients commonly require incisional glaucoma surgery earlier in the treatment algorithm, and despite that they may still do poorly—this mantra is commonly taught for the management of angle-recession glaucoma. However, it’s based on only a few single-center retrospective studies with small sample sizes.
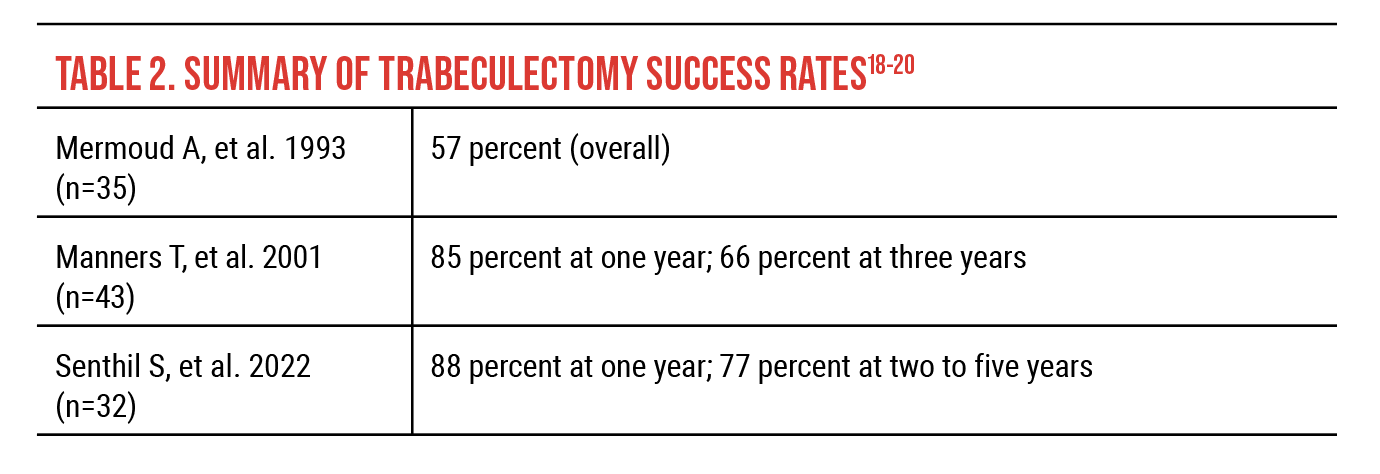
Before mitomycin-C was routinely used with trabeculectomy, a 1993 study reported 43-percent treatment success and 0-percent long-term success after six years in angle-recession glaucoma.17 With antimetabolites, failure rates of roughly 50 percent two to three years after incisional surgery were reported in older studies. As a secondary procedure, trabeculectomy plus antimetabolite was successful in four of seven cases (57 percent) in a retrospective 1993 study of 65 patients undergoing drainage procedures.18 Among tube shunt procedures in the same study, success rates were 56 percent at one year and 27 percent at five years using Molteno single-plate implantation (n=20).18
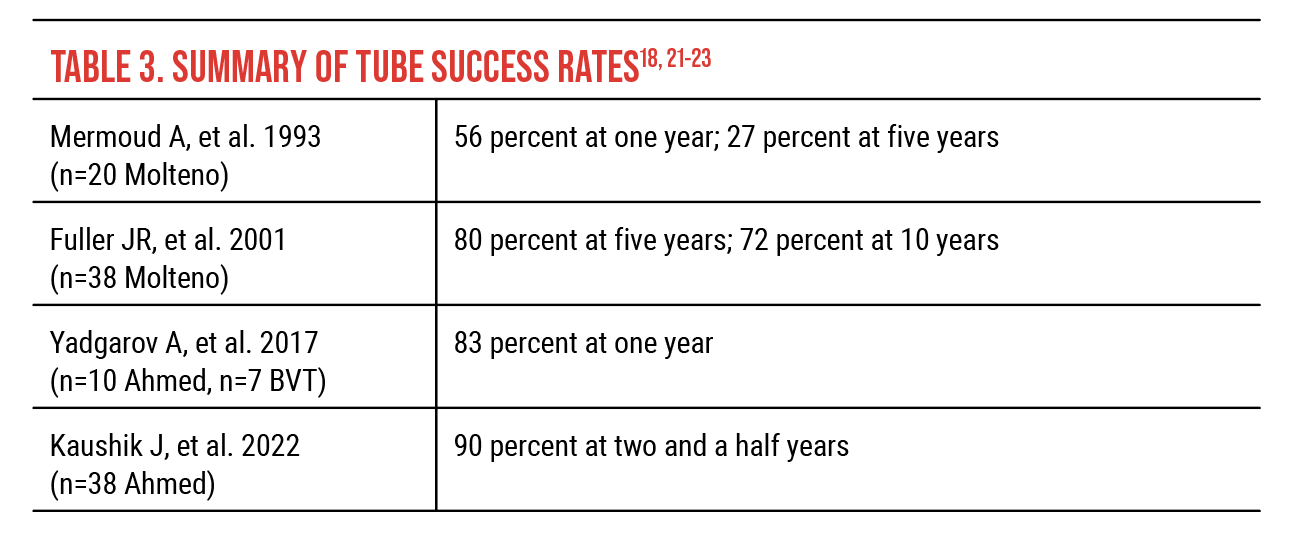 |
These are all small studies with significant limitations to them, but more recent surgical results from other single-center retrospective studies demonstrate that the outcomes are better than what we’ve traditionally believed (Tables 2 and 3). A 2001 retrospective study of 43 trabeculectomy procedures using mitomycin-C reported 85-percent success at one year and 66-percent success at three years.19 In 2022, a study of 32 eyes reported complete survival of trabeculectomy with mitomycin-C in 88 percent of eyes at one year and 77 percent two to five years postoperatively.20
In 2001, a study reported Molteno success rates for 38 procedures of 80 percent at five years and 72 percent at 10 years.21 Success rates reported in 2017 for Ahmed (n=10) and Baerveldt (n=7) tubes were 83 percent at one year.22 In 2022, a study reported a 90-percent success rate for Ahmed tubes at two-and-a-half years (n=38).23
With the caveat that failure and inclusion criteria differ, the outcomes reported in these small retrospective studies surprisingly show higher success rates than those reported in our largest randomized trials, the Tube Versus Trabeculectomy Study,24-25 the Ahmed Versus Baerveldt Study and the Ahmed Baerveldt Comparison Study,26 which demonstrated failure rates at five years between 34 and 49 percent, with more than a quarter of patients requiring additional procedures within five years.27 Whether these higher success rates in the smaller studies hold true in clinical practice remains to be seen, but the more recent, albeit limited, literature we have is a bit more encouraging. I think the takeaway is that the literature doesn’t give us the same impression as what’s taught, nor is the literature as conclusive as we tend to think when it comes to treating angle-recession glaucoma with tube shunts and trabeculectomies.
• MIGS. Considering that minimally invasive glaucoma surgeries are relatively new procedures, there’s little in the literature about their use in angle-recession glaucoma. However, a retrospective single-center study published in 2022 found that penetrating canaloplasty may be an effective treatment option.28 The study included 40 eyes of 40 patients with angle recession and reported success rates of 87.5 percent at six months and 89.5 percent at 12 months. Mean IOP decreased from baseline 37.8 ±12.3 mmHg to 14.8 ±3.6 mmHg on 0.1 ±0.5 medications at 12 months postop (p<0.05).
The authors advocate that if MIGS is an option, an angle penetrating surgery—either an ab externo or ab interno penetrating canaloplasty—should be performed, rather than a stenting procedure or a non-penetrating or limitedly penetrating angle-based procedure, based on the presumed mechanism of a functional obstruction of the trabecular meshwork.
Importantly, if performing MIGS on an angle-recession patient, carefully monitor the frequency and duration of postoperative topical steroids because these patients may be more prone to an IOP spike. In 1967, George Spaeth, MD, demonstrated a correlation between steroid responsiveness and angle recession,29 and a more recent study published in 2022 found that patients with angle recession were much more likely to have a steroid response (defined as an IOP increase >5 mmHg beginning at least three days after surgery) after MIGS.30
• Cyclodestructive procedures. Patients with more severe and advanced glaucoma may be amenable to a cyclodestructive procedure such as transscleral laser or potentially endoscopic cyclophotocoagulation.
In summary, trauma is likely a much more common cause of glaucoma than we think. Many patients forget they’ve had trauma unless it was very significant, but even minor trauma can cause this disease, so be sure to perform a good and careful gonioscopy in these patients and maintain suspicion for angle-recession glaucoma, especially if the presentation is asymmetric. Additionally, since angle-recession glaucoma remains a more difficult disease to manage than open-angle glaucoma, it’s important to counsel patients appropriately so they’re aware of what they may be facing. Lastly, consider SLT in appropriate patients, especially if the next step on the decision tree is surgery.
Dr. Moore is an associate professor of ophthalmology at the University of Kentucky. He has no related financial disclosures.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Wolff SM, Zimmerman LE. Chronic secondary glaucoma. American Journal of Ophthalmology 1962;54:4:547-563.
2. Collins ET, Edward T. University College LLS. On the pathological examination of three eyes lost from concussion. London: Ophthalmological Society of the United Kingdom 1892.
3. Pujari A, Selvan H, Behera AK, et al. The probable mechanism of traumatic angle recession and cyclodialysis. Journal of Glaucoma 2020;29:1:67-70.
4. Herschler J. Trabecular damage due to blunt anterior segment injury and its relationship to traumatic glaucoma. Trans Am Acad Ophthalmol Otolaryngol 1977;83:239.
5. Mooney D. Angle recession and secondary glaucoma. Br J Ophthalmol 1973;57:8:608–612.
6. Mansoori T, Reddy AA, Balakrishna N. Identification and quantitative assessment of Schlemm’s canal in the eyes with 360° angle recession glaucoma. J Curr Glaucoma Pract 2020;14:1:25–29.
7. Tonjum AM. Intraocular pressure and facility of outflow late after ocular contusion. Acta Ophthalmol 1968;46:5:886–908.
8. Blanton FM. Anterior chamber angle recession and secondary glaucoma: A study of the after effects of traumatic hyphemas. Arch Ophthalmol 1964;72:1:39–44.
9. Howard GM, Hutchinson BT, Fredrick AR Jr. Hyphema resulting from blunt trauma: Gonioscopic, tonographic, and ophthalmoscopic observation following resolution of the hemorrhage. Trans Am Acad Ophthalmol Otolaryngol 1965;69:294e306.
10. Sihota R. Early predictors of traumatic glaucoma after closed globe injury: Trabecular pigmentation, widened angle recess, and higher baseline intraocular pressure. Arch Ophthalmol 2008;126:7:921.
11. Alper MG. Contusion angle deformity and glaucoma. Arch Ophthalmol 1963;69:4:455–467.
12. Bleiman BS, Schwartz AL. Paradoxical intraocular pressure response to pilocarpine: A proposed mechanism and treatment. Arch Ophthalmol 1979:97;1305-6.
13. Goldberg I. Argon laser trabeculoplasty and the open-angle glaucomas. Australian and New Zealand Journal of Ophthalmology 1985;13:3:243-248.
14. Robin AL, Pollack JP. Argon laser trabeculoplasty in secondary forms of open-angle glaucoma. Arch Ophthalmol 1983;101:3:382-4.
15. Chang TC, Vanner EA, Fujino D, et al. Factors associated with laser trabeculoplasty response duration: Analysis of a large clinical database (IRIS Registry). Journal of Glaucoma 2021;30:10:902-910.
16. Chang TC, Parrish RK, Fujino D, Kelly SP, Vanner EA. Factors associated with favorable laser trabeculoplasty response: IRIS Registry analysis. American Journal of Ophthalmology 2021;223:149-158.
17. Mermoud A, Salmon JF, Straker C. Post-traumatic angle recession glaucoma: A risk factor for bleb failure after trabeculectomy. Br J Ophthalmol 1993;77:631-4.
18. Mermoud A, Salmon JF, Barron A, et al. Surgical management of post-traumatic angle recession glaucoma. Ophthalmol 1993;100:5:634-42.
19. Manners T, Salmon JF, Barron A, et al. Trabeculectomy with mitomycin C in the treatment of post-traumatic angle recession glaucoma. Br J Ophthalmol 2001;85:159–163.
20. Senthil S, Dangeti D, Battula M, et al. Trabeculectomy with mitomycin C in post-traumatic angle recession glaucoma in phakic eyes with no prior intraocular intervention. Semin Ophthalmol 2022;37:2:171-6.
21. Fuller JR, Bevin TH, Molteno ACB. Long-term follow-up of traumatic glaucoma treated with Molteno implants. Ophthamlology 2001;108:1796-1800.
22. Yadgarov A, Liu D, Crane ES, et al. Surgical outcomes of Ahmed or Baerveldt tube shunt implantation for medically uncontrolled traumatic glaucoma. J Curr Glaucoma Pract 2017;11:1:16-21.
23. Kaushik J, Parihar JKS, Sing A, et al. Evaluation of primary Ahmed glaucoma valve implantation in post-traumatic angle recession glaucoma in Indian eyes. Int Ophthalmol 2022;42:3:817-27.
24. Gedde SJ, Schiffman JC, Feuer WJ, on behalf of the Tube versus Trabeculectomy Study Group. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012;153:789-803.
25. Gedde SJ, Feuer WJ, Lim SH, Primary tube versus trabeculectomy study group. Treatment outcomes in the primary tube versus trabeculectomy (PTVT) study after 5 years of follow-up. Ophthalmology 2022;129:12:1344-56.
26. Christakis PG, Zhang D, Budenz DL, ABC-AVB Study Groups. Five-year pooled data analysis of the Ahmed Baerveldt Comparison Study and the Ahmed Versus Baerveldt Study. Am J Ophthalmol 2017;176:118-26.
27. Craven ER, Singh IP, Yu TM, et al. Reoperation rates and disease costs for primary open-angle glaucoma patients in the United States treated with incisional glaucoma surgery. Ophthalmol Glaucoma 2022;5:3:297-305.
28. Cheng H, Ye W, Zhang S, et al. Clinical outcomes of penetrating canaloplasty in patients with traumatic angle recession glaucoma: A prospective interventional case series. Br J Ophthalmol. Published online March 22, 2022.
29. Spaeth GL. Traumatic hyphema, angle recession dexamethasone hypertension, and glaucoma. Arch Ophthalmol 1967;78:6:714-21.
30. Abtahi M, Rudnisky CJ, Nazarali S, Damji KF. Incidence of steroid response in microinvasive glaucoma surgery with trabecular microbypass stent and ab interno trabeculectomy. Canadian Journal of Ophthalmology 2022;57:3:167-174.



