 |
The Type 1 Boston keratoprosthesis, better known as a KPro, is a unique device indicated for severe ocular pathology. Though it’s a boon for many patients, the KPro can make dealing with co-existing glaucoma more challenging. How often you’ll encounter a patient with a KPro may depend on the nature and location of your practice: If you have a solo practice somewhere remote, you may not see many of these patients, but if you work at an academic center where your cornea colleagues are regularly performing these surgeries, you’re likely to see a number of them.
Here, I’ll discuss the issues you need to be prepared to deal with when you encounter a KPro patient—especially issues relating to glaucoma.
The KPro’s Design
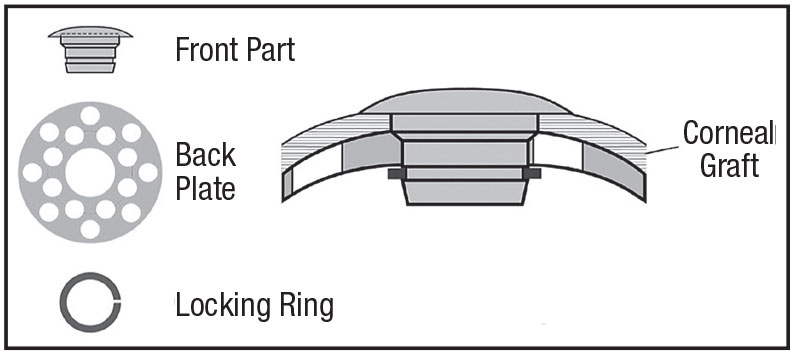
The device itself consists of a plastic front plate with a stem that passes through a corneal tissue graft, with a back plate and a locking ring that holds the device together. (See illustration.) The assembled device is sutured in place much as you would normally suture the donor graft during a penetrating keratoplasty.
A KPro implant is generally considered a last-resort option for patients with corneal opacification associated with a number of indications, such as repeated corneal graft failure, ocular trauma, herpetic keratitis, limbal stem cell deficiency, aniridia, Stevens-Johnson Syndrome and con-genital opacification;1 fortunately, it successfully rehabilitates vision in many of these cases. One paper reported that as of the summer of 2014, more than 9,000 KPros had been implanted throughout the world. That was up from 1,161 in 2009, so the number is clearly increasing.1
The Glaucoma Factor
Glaucoma is often an issue with these eyes, both before a KPro is implanted and after. Glaucoma is al-ready prevalent in up to 73 percent of these patients before receiving a KPro. This is often related to underlying ocular pathology (herpetic keratitis, aniridia, ocular trauma, etc.).2
Even if they don’t have glaucoma when they receive the KPro, up to 64 percent of these patients will develop glaucoma afterward.3 This may be partly a result of being at high risk for glaucoma prior to the implant, but it can also be triggered by the presence of the device. One possible reason for this may be that there can be progressive narrowing of the angle after these implants go in.4 That, in turn, may lead to the development of synechiae, further closing off the angle. In any case, these patients’ glaucoma tends to be pretty aggressive; up to 23 percent will experience glaucoma progression.5 In fact, it’s a leading cause of vision loss in these patients.6
Prior to KPro implantation, evaluating the patient for existing glaucoma is helpful. Unfortunately, this can be challenging to do; sometimes the cornea is opaque. But to whatever extent you can, you should try to get a picture of the optic nerve, try to assess the visual field and get an OCT. Any kind of baseline evaluation you can obtain will help you know where you’re starting.
Some research suggests that if there’s any evidence of glaucoma before the KPro surgery, you should strongly consider a combined KPro and glaucoma surgery, because we know the glaucoma-related risks are so high after the prosthesis goes in and we know our methods of monitoring glaucoma may be limited afterwards. Addressing glaucoma in this situation usually means implanting a glaucoma drainage device; the effectiveness of this approach was first demonstrated by Peter Netland, MD, and Claes Dohlman, MD, PhD, back in 1998.7 These can be fairly extensive surgeries, and if you proceed with a combined surgical approach, you may need to coordinate with your cornea or retinal colleagues to create the surgical plan.
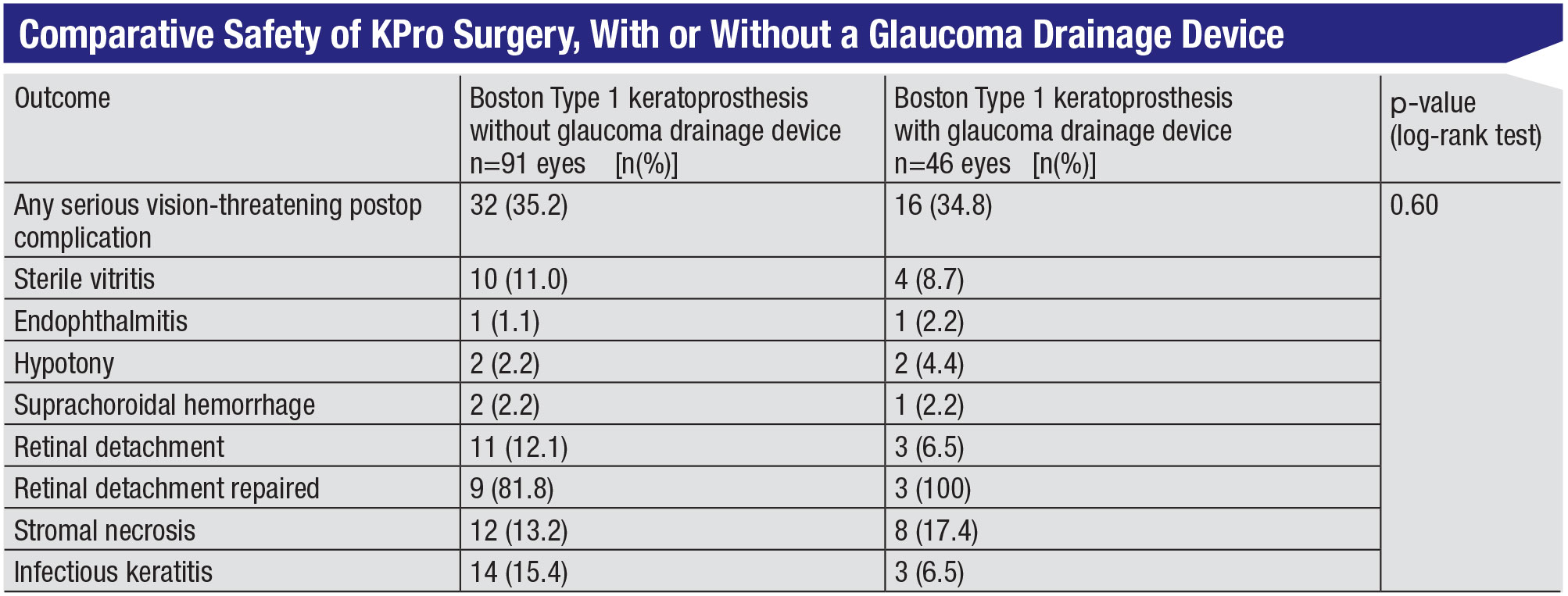
Of course, a valid concern is whether combining these surgeries is safe for the patient. A 2017 retrospective study from the Jules Stein Eye Institute at UCLA compared KPro surgery alone to KPro surgery combined with glaucoma surgery; no significant differences in the number of vision-threatening complications were found.4
 |
Tube Shunt Strategies
If you do find yourself implanting a tube shunt in a KPro patient, these strategies will help you achieve a successful outcome:
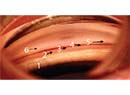
• Consider putting the tube in the sulcus or the pars plana. The anterior chamber in these eyes tends to shallow or collapse, leading to a lot of angle closure and synechiae. For that reason you may want to consider putting the tube in the sulcus or even the pars plana to minimize the risk of it becoming occluded.
• Make sure the tube is long and radially positioned. This can help you visualize the tip of the tube in the postoperative period more easily.
• Consider inserting the tube as posteriorly as you can, possibly with a long scleral tunnel, to minomize the erosion risk. Some of these patients chronically wear bandage contact lenses, and having a contact lens riding right over the tube insertion site may facilitate erosion.Also, the tube has to be inserted into the eye more posteriorly because of the back plate on the keratoprosthesis.
• Consider using a scleral or corneal patch graft rather than pericardium. Some authors have hypothesized that using pericardium may be associated with higher rates of erosions with these patients.8
If the patient already has a tube shunt, but the pressure-lowering has been inadequate, you may decide to implant a second tube shunt or perform cyclophotocoagulation. (Of course, this still requires going through the steps of assessing how stable and well-controlled the glaucoma is.) The presence of the previous shunt shouldn’t impact your surgery, unless the original tube was placed very anteriorly. In that situation, you might need to reposition the tube so it’s more posterior to try to minimize the risk of erosion.
 |
Evaluating a KPro Eye
Because the risk of glaucoma is so great after a KPro, it’s essential to monitor these patients. However, this may not be easy. One of the biggest challenges is figuring out how to measure the IOP, because you can’t applanate plastic. Traditionally we’ve relied on digital palpation, where you gently bounce the globe between your two fingers under the closed eyelid, to estimate the IOP. But the accuracy of this approach varies based on examiner experience; studies have shown that 31 percent of measurements may be off by as much as 30 percent.9,10
Some studies have looked at other, possibly more reliable ways of measuring the pressure. These include:
• Scleral Schiotz tonometry. This was one of the first ways ophthalmologists measured intra-ocular pressure; it’s been around for more than 100 years. A lot of third-world countries that don’t have access to more modern equipment still use it. In fact, one study concluded that scleral Schiotz tonometry has the highest accuracy when measuring IOP in patients with a keratoprosthesis, compared to the Tonopen and gold-standard digital manometry.11
Another study conducted last year looked at scleral Schiotz tonometry vs. Goldmann applanation. The study authors developed a formula to convert the scleral Schiotz pressure to an equivalent Goldmann pressure and then tested the formula on a series of patients in their clinic. They found that the agreement between the measurements was fairly good; the mean bias was less than -1 and the limits of agreement were pretty reasonable—about -3.7 to 1.8, which is quite good when comparing two IOP devices.12
Of course, some clinics may not have this equipment any longer. However, the device is inexpensive—you can still purchase them, typically for less than $100.
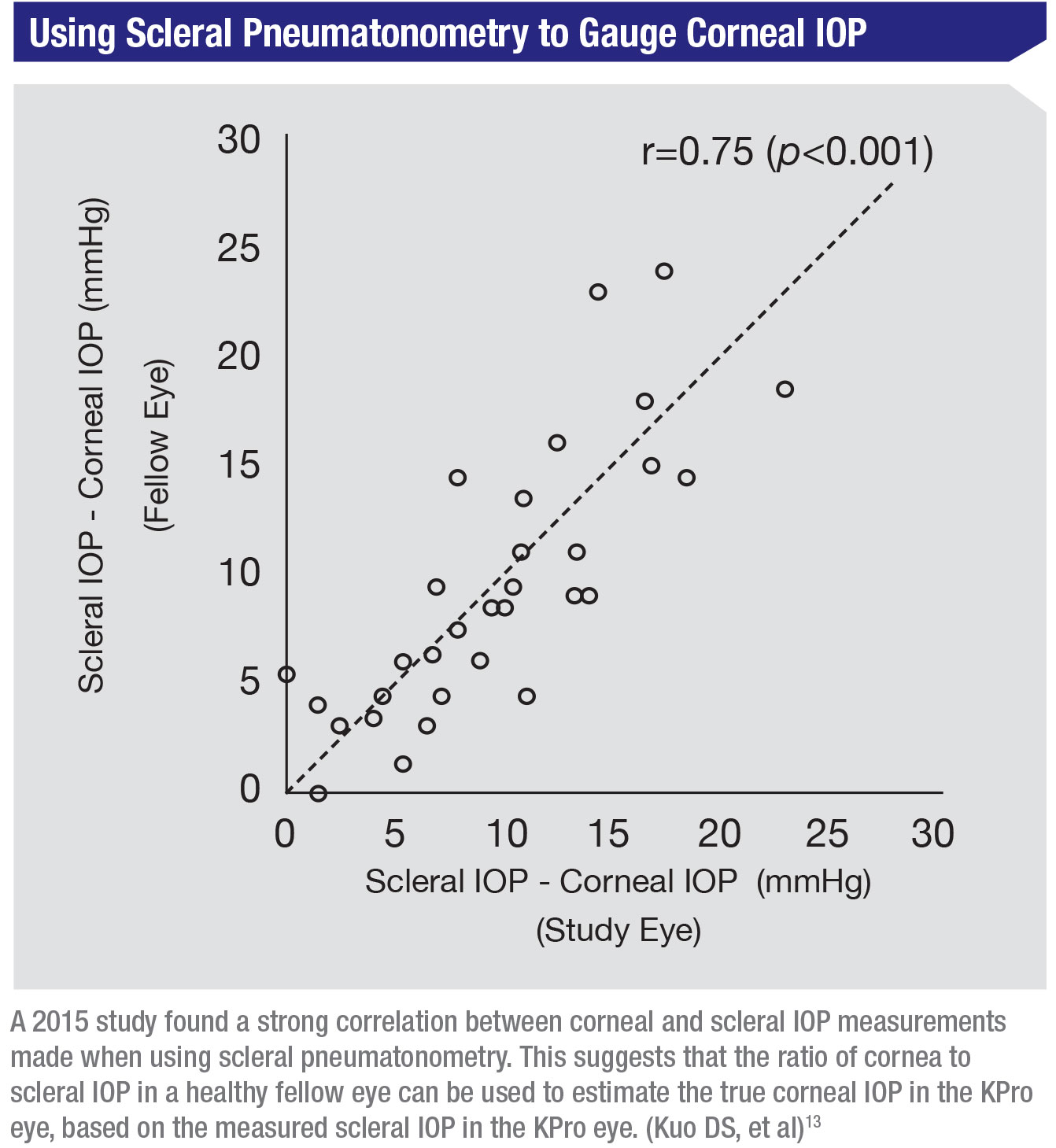
• Scleral pneumatonometry. This is probably more commonly used in this situation than scleral Schiotz tonometry. Two studies, out of UCSF and UCLA, measured the temporal sclera using the pneumatonometer probe and compared it to the corneal pressure measurement. Both of these studies came up with formulas to convert the scleral IOP measurement to a corneal IOP.13,14 This approach is pretty helpful in the clinic; sometimes I’ll use it and do a rough conversion just to get an idea how the patient is doing.
One thing I like about the study out of UCSF13 is that they did a comparison between the corneal and scleral IOP measurements of paired eyes on the same patient. (See graph, above.) The differences were highly correlated. That means that in practice, you can estimate the corneal pressure in the KPro eye based on the scleral measurement, using the difference in the measurements you find in the healthier eye.
The other nice thing they did in this study was to monitor the pressure in patients who had just got-ten intravitreal anti-VEGF injections, which are known to cause transient eye pressure elevations. They measured both the cornea and sclera of each eye as the pressure was returning to normal, collecting serial pressure measurements. The differences between scleral and corneal measurements tended to re-main constant, even as the pressures were changing. This suggests that you can use the scleral IOP measurement to monitor the eye with a KPro over time, and that scleral IOP changes are representative of corneal IOP changes.
• Implantable sensors. As technology advances, implantable sensors are becoming more sophisticated. Last year researchers reported placing the Eyemate-IO sensor in KPro eyes; they found that there was a good correlation between the telemetric IOP and tactile palpation.15,16
To monitor a KPro patient’s eye for glaucoma or glaucoma progression, you also have to assess optic nerve function and structure. Getting a visual field can be challenging, because the KPro optic limits your visual field to about 90 or 95 degrees. You can still do a Humphrey visual field, but sometimes the testing is unreliable in these patients. For that reason you may want to consider monitoring with Goldmann visual field testing. Studies have demonstrated that this can be effective for monitoring glaucoma progression in these patients.17
In terms of assessing structure, again, the 3-mm optic can make things difficult to visualize. If you’re trying to get a photo of the disc, a nonmydriatic camera can be helpful. If you’re using OCT, the KPro can cause segmentation errors, which you need to be aware of when interpreting the result. Meanwhile, new approaches continue to evolve. At the Massachusetts Eye and Ear Infirmary they’ve developed a 3-D OCT volumetric scan protocol that seems to be effective for monitoring structural changes in these patients.
If You Add CPC …
So what do you do if you need additional IOP control after the KPro is in place? If the patient al-ready has a drainage tube, you could certainly consider a second tube, but many surgeons will try cyclophotocoagulation.
A few studies have looked at cyclophotocoagulation in KPro eyes. They found a fairly good rate of success controlling glaucoma following CPC
—61 to 67 percent—and a relatively low complication rate.18,19 What’s really interesting is that both studies reported cases of endophthalmitis, which we don’t normally associate with cyclophotocoagulation since it’s not an incisional procedure. A possible explanation for this is that the junction between the optic and the corneal graft never fully integrates—after all, it’s a junction of plastic and biological material. If that’s the case, organisms could theoretically enter the eye at that interface if something causes the interface to gape.
Since this is a potential problem, some specific techniques should be followed:
• Treat this as an intraocular surgery. Consider using aseptic technique, including betadine prep.
• Transilluminate the eye. The anatomy in these eyes can be a little distorted; you can get disoriented during surgery because the landmarks are often difficult to visualize. Trans-illuminating the eye can help you identify the ciliary body bands, so you can make sure you’re treating the right area.
• Avoid pushing on the eye. Pushing on the eye can cause the interface to gape, increasing the risk of endophthalmitis.
• Monitor for hypotony afterwards. Hypotony could also theoretically cause the interface to gape and increase the patient’s infection risk.
A Battle Worth Winning
If you’re performing the KPro surgery yourself, re-member that glaucoma is very common in these eyes, and consider implanting a tube shunt before or at the time of KPro surgery. If you’re managing a patient with a KPro implant, you may find that monitoring glaucoma remains challenging, but it’s not impossible. Meanwhile, newer technology on the horizon will eventually improve our ability to help this challenging population.
Some of these patients are extremely complex, but they can also be some of your most satisfying cases. REVIEW
Dr. Wen is a glaucoma specialist at the Duke Eye Center and an associate professor of ophthalmology at Duke University School of Medicine. She reports no financial interest in any products discussed in this article.
1. Lee WB, Shtein RM, Kaufman SC, et al. Boston Keratoprosthesis: Outcomes and Complications: A report by the American Academy of Ophthalmology. Ophthalmology 2015;122:7:1504-11.
2. Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea 2009;28:9:989-96.
3. Lee WB, Shtein RM, Kaufman SC, et al. Boston Keratoprosthesis: Outcomes and Complications: A report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:7:1504-11.
4. Silva RNE, Taniguchi EV, et al. Angle anatomy and glaucoma in patients with Boston keratoprosthesis. Cornea 2020;39:6:713-719.
5. Lenis TL, Chiu SY, et al. Safety of concurrent Boston type I keratoprosthesis and glaucoma drainage device implantation. Ophthalmology 2017;124:1:12-19.
6. Nguyen P, Chopra V. Glaucoma management in Boston keratoprosthesis type I recipients. Curr Opin Ophthalmol 2014;25:2:134-40.
7. Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology 1998;105:4:751-7.
8. Marie-Claude Robert MC, Pomerleau V, Harissi-Dagher M. Complications associated with Boston keratoprosthesis type 1 and glaucoma drainage devices. Br J Ophthalmol 2013;97:5:573-7.
9. Baum J, Chaturvedi N, Netland PA, Dreyer EB. Assessment of intraocular pressure by palpation. Am J Ophthalmol 1995;119:5:650-1.
10. Rubinfeld RS, Cohen EJ, et al. The accuracy of finger tension for estimating intraocular pressure after penetrating keratoplasty. Ophthalmic Surg Lasers 1998;29:3:213-5.
11. Estrovich IE, Shen C, Chu Y, et al. Schiotz tonometry accurately measures intraocular pressure in Boston type 1 keratoprosthesis eyes. Cornea 2015;34:6:682-5.
12. Senthil S, Chary R, Ali MH, et al. Schiotz scleral intraocular pressure readings predict Goldmann applanation readings better than rebound tonometry. Cornea 2019;38:9:1117-1123.
13. Kuo DS, Ou Y, Jeng BH, et al. Correlation of serial scleral and corneal pneumatonometry. Ophthalmology 2015;122:9:1771-6.
14. Kapamajian MA, de la Cruz J, Hallak JA, et al. Correlation between corneal and scleral pneumatonometry: An alternative method for intraocular pressure measurement. Am J Ophthalmol 2013;156:5:902-906.e1.
15. Enders P, Hall J, Bornhauser M, et al. Telemetric intraocular pressure monitoring after Boston keratoprosthesis surgery. Ophthalmology 2019;126:2:322-324.
16. Enders P, Hall J, Bornhauser M, Mansouri K, et al. Am Telemetric intraocular pressure monitoring after Boston keratoprosthesis surgery using the Eyemate-IO Sensor: Dynamics in the first year. J Ophthalmol 2019;206:256-263.
17. Ali MH, Dikopf MS, Finder AG, et al. Assessment of glaucomatous damage after Boston keratoprosthesis implantation based on digital planimetric quantification of visual fields and optic nerve head imaging. Cornea 2018;37:5:602-608.
18. Rivier D, Paula JS, Kim E, Dohlman CH, Grosskreutz CL. Glaucoma and keratoprosthesis surgery: Role of adjunctive cyclophotocoagulation. J Glaucoma 2009;18:4:321-4.
19. Jabbour S, Harissi-Dagher M, Agoumi Y, et al. Cyclophotocoagulation in the control of glaucoma in patients with the Boston keratoprosthesis type 1. Cornea 2020;39:2:181-185.