When phakic IOLs first hit the market, there was an undeniable level of enthusiasm about the promise of improving vision for those with high refractive errors but who weren’t candidates for LASIK or PRK. Yet, years later, many surgeons—especially in the United States—haven’t embraced them for reasons ranging from patient cost to associated risks and the success of LASIK.
 |
| The EVO/EVO+ ICL from Staar Surgical contains a hole in its center, designed to improve aqueous humor circulation in the eye, thereby eliminating the need for an iridotomy. |
Perhaps contributing to the slow adoption of phakic IOLs was the fact that there have been only two FDA-approved options available: the Staar Visian ICL and the Ophtec Verisyse/Artisan. A toric version of the ICL was also approved in 2018. However, both of these options required the creation of a peripheral iridotomy, an extra step that discouraged surgeons from using phakic IOLs.
That hesitation may change now that the FDA has approved the Staar EVO/EVO+ Visian ICL and EVO/EVO+ Visian Toric ICL, each of which come with a hole in its center called the KS-AquaPort. According to Staar, the hole is designed to improve aqueous humor circulation in the eye, thereby eliminating the need for an iridotomy.
Benefits of the Central Port
Although considered safe, laser peripheral iridotomy complications have been known to occur, including transient blurred vision, intraocular pressure rise, dysphotopsia, hyphema, closure of the iridotomy and damage to other tissues.1
Neda Shamie, MD, based in Los Angeles, says the EVO is exciting for many reasons. “We’ve had experience with the implantable contact lens for many, many years, and the EVO is the same type we’ve had access to, but now it has a small opening in the center that essentially removes the need for us to perform a peripheral iridotomy,” she says. “So much of the challenge in placing the implantable contact lens was that surgeons didn’t want to do that peripheral iridotomy because it had its own inherent risks.”
Dr. Shamie says phakic IOLs appeal to patients because no tissue is being taken out and the lenses can be removed. “Patients see implantable contact lenses as an additive procedure,” she says. “You’re adding a contact lens, not removing tissue, and it’s essentially reversible. Performing an iridotomy really canceled out one of the main benefits of the ICL, which is that it can be taken out, while an iridotomy is a permanent defect in the peripheral iris that could potentially have complications associated with it.”
This reasoning could also be applied to position the ICL as an alternative to LASIK, says John Vukich, MD, who’s based in Wisconsin. “LASIK contours the cornea to create a new refraction, but it does so by removing tissue, and there’s a functional and practical limit to how much tissue you can remove and still maintain the structural integrity of what’s left behind. That’s why high levels of nearsightedness can’t be treated by LASIK: It requires too much tissue removal. The ICL, however, doesn’t remove anything. Once you’ve had LASIK, there’s no ability to restore the cornea to its previous condition, but the ICL is removable, and some patients are motivated by that—although it’s extremely rare that a patient finds something they don’t like about the ICL.”
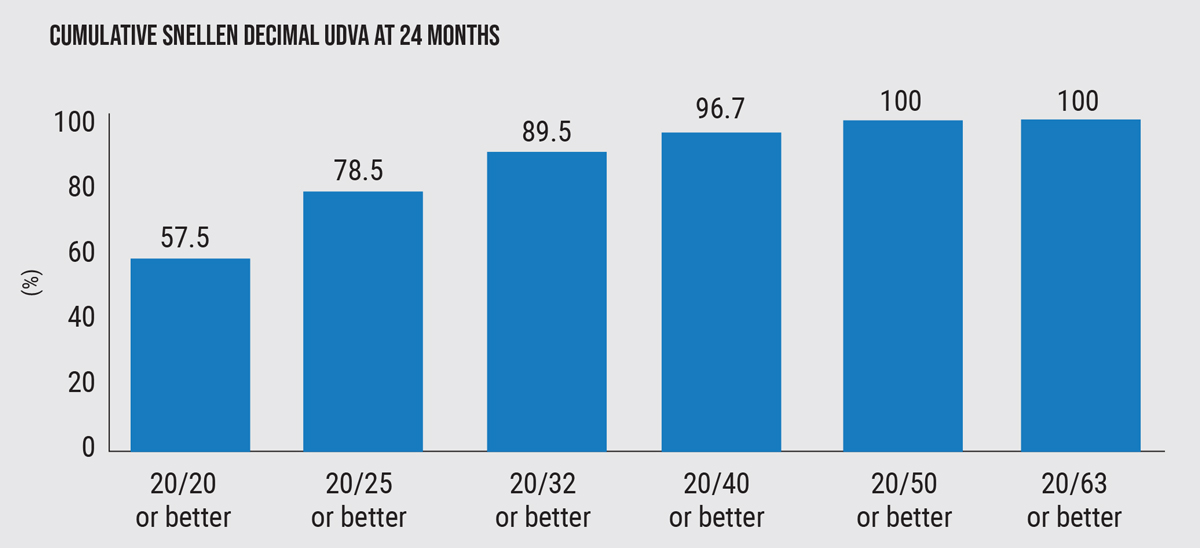 |
| Figure 1. The cumulative proportion of eyes having a given UDVA value at 24 months following implantation of a collamer lens with a central hole. Before surgery, all eyes had an UDVA worse than 20/63. |
The Ideal Patient and Outcomes to Expect
The EVO Visian ICL is available for the correction or reduction of myopic astigmatism in patients with spherical equivalents ranging from -3 to -20 D, with astigmatism from 1 D to 4 D at the spectacle plane; with an anterior chamber depth of at least 3 mm when measured from the corneal endothelium to the anterior surface of the crystalline lens; and a stable refractive history (defined as not varying more than 0.5 D for one year prior to implantation).
According to Staar, a million EVO procedures have already been performed worldwide. U.S. surgeons have been hearing about the EVO’s success from their international peers for years, says Dr. Vukich. “The EVO model has been available for several years in virtually every country except the U.S. It’s a very popular device, especially in Asia, where the average number of individuals with myopia is higher than in Western countries,” he says.
A clinical study followed 327 patients with either the EVO/EVO+ Visian ICL or the EVO/EVO+ Visian Toric ICL. A total of 75.9 percent of patients reported 20/20 vision or better in the implanted eye, and 98.9 percent had 20/32 vision in the implanted eye after six months.2
The FDA advises not to use the EVO/EVO+ on anyone who is pregnant or nursing, younger than 21, has moderate to severe glaucoma, has shallow space in the anterior chamber or a small anterior chamber angle, or whose endothelial cell density falls outside of a specified range based on their age and the size of ICL being implanted.
Dr. Vukich has implanted the EVO in individuals who are unable to wear contact lenses. “One patient couldn’t wear them and the other developed an allergic reaction and had to give up wearing them,” he says. “They were extremely motivated, but corneal refractive surgery wasn’t for them, so these patients waited several months for the EVO to come in. They are just thrilled to be able to have that.”
Surgical Tips and Adverse Effects
Staar Surgical says the EVO implantation takes less than 30 minutes to perform.
Dr. Vukich says the surgery isn’t overly complicated. “It’s absolutely approachable and within the skill set of any anterior segment surgeon,” he says. “If a surgeon is already familiar with the precursor, the standard ICL, there’s no learning curve. It’s identical to how the lens sits and handles; this is simply a better version. For surgeons who are new to the ICL, it’s straightforward and can be done through a 2.6- or 2.8-mm clear corneal incision. For a surgeon who’s comfortable with intraocular surgery and comfortable doing a cataract operation, the ICL is significantly less involved and requires fewer steps than a cataract operation.”
One thing Dr. Shamie has learned is to ensure that all the viscoelastic is removed and to keep the pupil dilated. “If there’s any viscoelastic trapped behind the ICL you want to give it time to circulate out from behind it so that the central hole will be patent and allow for aqueous flow,” she says.
The EVO/EVO+ ICL study did require an IOP check at one to six hours postoperatively, which hadn’t been done with the previous ICL models. The study found a significant rate of IOP spikes, approximately 19.9 percent, which appear to have been related to incomplete removal of the dispersive OVD at the end of the procedure. These day-zero IOP increases were resolved with medication and/or paracentesis/AC tap.2
Dr. Shamie uses multiple modes to measure the white-to-white and calculate the best ICL size. “Our approach is to measure the white-to-white using our biometer, slit lamp and UBM,” she says. “We compare the lens size calculated by each modality and decide accordingly. I have the greatest confidence in our UBM measurements, but I do look at the average. If the sizing for the ICL lands the patient between two sizes, I err on the smaller size with the EVO ICL because the small opening in the optic of the EVO lessens the risk of cataract formation, so there’s less concern about going too small.”
During conversations with patients, Dr. Vukich makes sure they know that there’s still an inherent risk of retinal detachment and a higher risk of cataracts at a younger age. “That’s not because they did or didn’t have the ICL, it’s just part of being myopic,” he says. “I also tell them that, rarely, there could be a little bit of glare at night, especially for the very high nearsighted individuals. It’s unusual and not everyone has it, and when they do it’s really minimal.”
Both Drs. Vukich and Shamie believe the EVO will help make phakic IOLs a more popular choice in an ophthalmologist’s arsenal.
“ICLs have evolved and continue to gain traction around the world, and now we can offer them to patients with this added safety profile,” says Dr. Vukich.
Dr. Chayet is considered a pioneer in refractive and cataract surgery, and is the medical director of the Codet Vision Institute in Tijuana, Mexico. He is a clinical investigator for RxSight, LensGen and ForSight Vision6. Drs. Vukich and Shamie are consultants for Staar.
1. Kam JP, Zepeda EM, Ding L, et al. Resident-performed laser peripheral iridotomy in primary angle closure, primary angle closure suspects, and primary angle closure glaucoma. Clin Ophthalmol 2017;11:1871–1876.
2. EVO/EVO+ VISIAN Implantable Collamer Lens – P030016/S035. FDA.gov. Accessed May 3, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030016S035C.pdf.