Often, a diagnosis is entirely dependent on the doctor's perspective. When a person experiences itchy, red eyes with congestion, sneezing and a runny nose, the diagnosis and treatment may hinge on whose office the patient visits: Primary care physicians and allergists are likely to diagnose allergic rhinitis (AR) with an ocular component, while ophthalmologists are likely to diagnose allergic conjunctivitis (AC) with a nasal component. At least 40 percent of seasonal AR patients also suffer from ocular itching and watery eyes,1 and some 95 percent of allergic conjunctivitis patients report co-existing allergic diseases, most often allergic rhinitis.2 More accurately, such patients represent the alternate, singular diagnosis of allergic rhinoconjunctivitis. Multiple explanations of why ocular allergy often accompanies nasal allergy have been proposed, the simplest of which is the close proximity of the two organs and their similar mucosal composition, allowing near simultaneous exposure to aggravating pollens. There is also the matter of the naso-lacrimal duct, which is a direct anatomical connection between the organs. The explanation least understood is the proposed nervous system connection governed by parasympathetic input and termed the naso-ocular reflex.
At the center of it all is a turf war among the systemic medications, topical intranasal sprays and topical ophthalmic medications involving their ability to control all the signs and symptoms of allergic rhinoconjunctivitis. In the world of indications, marketing and comparative studies, just how much relief can any single anti-allergy modality provide for patients with allergic rhinoconjunctivitis?
The Atopic March
The allergic condition is pervasive, and is seldom content with afflicting only one anatomical region at a time; individuals with one form of allergy are particularly inclined towards having another form of allergy. The condition is driven by both heredity and environment. Hence, the allergic tendency—and not an allergy to a specific allergen—is believed to be inherited. Atopic eczema, the itchy rash that often afflicts infants, is often the first manifestation in allergy-prone individuals.3 By the time a child is 5 to 7 years old, allergic asthma and AR commonly follow, especially if the parents themselves are atopic.4 This process has often been referred to as the "atopic march." The co-morbidity of allergic asthma and AR is well-covered in the literature, to the extent that a "one airway, one disease" paradigm has been upheld by the Allergic Rhinitis and its Impact on Asthma (ARIA) workshop.5
Related Conditions
Several published studies lend credence to the co-morbidity of allergic conjunctivitis with other allergic disorders. An analysis of more than 400 pediatric cases revealed that all children who had acute or chronic AC also had other allergic conditions, particularly allergic rhinitis, allergic asthma or eczema. Of the children with AC, 97 percent had concomitant AR.6 Unfortunately, few studies on the incidence of concomitant AC and AR exist, but at least one study reported similar findings in adults, with more than 80 percent of patients with AC also experiencing the signs and symptoms of AR.2
|
Connecting A to B, and Vice-versa
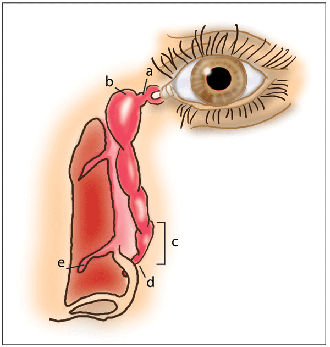
It's been suggested that the active drug, when sprayed up the nose, may reach the eye via the naso-lacrimal duct, where it may exert some therapeutic effects. There's no doubt that the eye and the nose are directly connected: When fluorescein is instilled in the eye, as it is during a Jones test, it's present in nasal secretions within five minutes, even after a nasal allergen challenge (NAC).15 The lacrimal duct is responsible for tear drainage and for providing the means of tear-film replenishment during the blink; it's continuous inferiorly with the naso-lacrimal duct. This duct eventually reaches the inferior meatus and empties about 5 mm posterior to the head of the inferior turbinate.16 Several valves are present in the lacrimal apparatus, among which are the valve of Rosenmüller, which prevents tear reflux, and the valve of Hasner. The valve of Hasner is a semi-perforate mucosal fold at the nasal opening of the naso-lacrimal duct.16 Unfortunately for nasal sprays, congestion and nasal blockage are primary symptoms of AR, which significantly inhibit drug delivery to the middle meatus. Inferior turbinate hypertrophy further contributes to nasal blockage.17 A variation of the Jones test, in which fluorescein was sprayed up the nose after a NAC and the eye was then examined for the presence of fluorescein, confirmed that little, if any, of what is sprayed up the nose reaches the eye.15
The Neural Connection
The eye is essentially a collecting plate for allergens, with little to protect it aside from the tear film and blink. Allergens and allergic mediators easily drain from the eye to the nose, provoking or exacerbating the nasal allergic reaction. The same study that utilized the Jones test to investigate the bi-directional patency of the naso-lacrimal duct also noted that the NAC does not induce clinically significant (one-unit difference) ocular allergic signs and symptoms. On the other hand, both the CAC and the NAC induced nasal symptoms to a comparable degree.15
An earlier study that examined the correlations among nasal challenges, nasal symptom scores and allergic mediators found that conjunctivitis can occur in approximately 20 percent of nasally provoked subjects.18 A neural reflex pathway between the eye and the nose has been suggested as a possible explanation for why nasal challenge may occasionally induce ocular signs and symptoms in some individuals.
It's well-established that chemical or mechanical stimulation of the nasal mucosa can result in lacrimation.19-21 Reflex tears in response to nasal or ocular irritation are the eye's attempt to wash the irritant out of both itself and the nasal lacrimal passage. This reflex is essentially a pain mechanism, similar to the tears stimulated by a stubbed toe. As such, individuals with autonomic nervous system disorders that decrease or diminish the ability to perceive pain, such as Riley-Day syndrome and congenital analgia, also have a diminished or complete inability to secrete tears.
One study, conducted in subjects with known ocular reactions to nasal challenge, reported that both nasal challenge with allergen and with placebo can induce itchy, watery eyes.22 These symptoms were significantly reduced with the administration of intranasal azelastine, but not with intranasal placebo. Ocular histamine levels were minimal or even undetectable after all challenges,22 confirming that the reduction in ocular symptoms wasn't due to binding of the antihistamine to H1 receptors in the eye. As histamine is the primary mediator of ocular allergy,23 there must be another explanation for the induced ocular itch and the reduction with the application of intranasal azelastine.
The lacrimal branch of the ophthalmic division of the fifth cranial nerve includes parasympathetic fibers. The cells of these fibers originate in the sphenopalatine ganglion, a parasympathetic cell station that ultimately links postganglionic fibers from the maxillary nerve to the lacrimal glands and to the glands of the nasal mucosa.21 Histamine is a neurotransmitter; it's therefore possible that intranasally applied histamine could induce an ocular sensation, and that an intranasally applied antihistamine could lessen it by blocking the activity of histamine in the nose. However, this potential reflex can't be interpreted as a primary therapeutic route to control clinically relevant signs and symptoms of AC.
Clinically Relevant Connections
Despite the lack of answers regarding the mechanism of the eye-nose treatment connection, repeated investigations of the effects of ophthalmic medications on the nose, and of intranasal medications on the eye, suggest that each treatment modality can provide some relief of the neighboring region.
Ophthalmic ketotifen14,24 and olopatadine 0.1%13 and 0.2%11 may significantly reduce the severity and frequency of nasal symptoms. In several instances, intranasal fluticasone furoate has demonstrated efficacy at reducing ocular signs and symptoms as quantified by a measurement known as the total ocular symptom score (TOSS);9,10 mometasone fumarate has been similarly investigated.12 Antihistamine nasal sprays, specifically intranasal azelastine22 and intranasal olopatadine,25 have both demonstrated significant reductions in ocular allergy symptoms.
These ancillary effects, however, haven't been consistently shown to be clinically relevant. Using the NAC and CAC, olopatadine 0.1% ophthalmic solution was compared to mometasone fumarate intranasal spray and to fexofenadine hydrochloride tablets for efficacy at controlling ocular and nasal signs and symptoms. Olopatadine 0.1% demonstrated statistically significant reductions in mean ocular itching scores post-CAC in comparison to the nasal spray and oral antihistamine; mometasone fumarate had similarly significant reductions in total nasal symptom scores (TNSS) post-NAC. While both olopatadine 0.1% and mometasone fumarate had some non-significant reductions in the symptoms of the neighboring regions, olopatadine 0.1% optimally controlled ocular allergy, while mometasone optimally controlled nasal allergy. Both topical medications consistently provided more relief to the local tissues than did the systemic antihistamine.15
The Measurement Toolbox
The methods typically used to evaluate the efficacies of intranasal sprays and ophthalmic anti-allergic medications are different. The CAC model utilizes standardized scales for grading each individual sign and symptom, and is routinely used for the evaluation of ophthalmic anti-allergic medications. In contrast, nasal sprays are typically evaluated in environmental models that utilize the TNSS, which groups all the nasal symptoms of AR into one scale. In pursuit of similar measurements of ocular signs and symptoms, a total ocular symptom score (TOSS) has been developed. These total symptom scores obscure the mechanism of action of the drug on the individual signs and symptoms, hindering direct drug comparisons.
Clinical trials of ophthalmic anti-allergic medications are required to show a one-unit difference (clinical relevance) between baseline and challenged scores for each primary sign and symptom (redness and itching) of AC. Nasal sprays don't currently have such guidelines on what's necessary to demonstrate clinically relevant effects on ocular signs and symptoms: Published trials show only statistically significant differences between drug and placebo for a composite score of total ocular symptom scores.
The Completed Picture
Many articles have attempted to address the complexities of the three modalities of allergy therapy—intranasal, ophthalmic and systemic medications. This area is of paramount interest to all clinicians. The chosen therapy must minimize side effects while maximizing the concentration delivered to the target tissue. We do know that treating topical disease topically is the best approach for safety and efficacy, based on the multiple studies that have described both the pharmacokinetics and the clinical efficacies of such medications.26,27
While intranasal therapies have demonstrated some ancillary effects in the eye, and ocular medications have demonstrated some effects in the nose, there's no doubt that it's clinically more effective to treat ocular signs and symptoms with ophthalmic drops.15,28 Similarly, it's clinically apparent that nasal signs and symptoms are most effectively treated with an intranasal spray. Systemic therapy has been shown to have mild effects on the eye and nose; however, intranasal sprays and ophthalmic drops provide more rapid relief of acute symptoms. Systemic therapy can enhance and complement the effects of ophthalmic drops and nasal sprays.26
Synergistic effects have been shown among the medications, particularly with concomitant dosing of ophthalmic and intranasal drugs.15,28,29 To this end, when discussing rhinoconjunctivitis, it can be expected that polypharmacy will produce enhanced effectiveness over monotherapy. Indeed, most allergic subjects consider effective treatment of their eye symptoms to be as important as treatment of their rhinorrhea. A long-acting ophthalmic antihistamine/mast cell stabilizer plus an intranasal corticosteroid or intranasal dual-acting antihistamine effectively treat all the signs and symptoms of rhinoconjunctivitis.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Mr. Gomes is the director of allergy, Ms. Lilyestrom is the managing editor, and Ms. Taylor is a medical writer at Ophthalmic Research Associates in North Andover.
1. Scadding GK, Richards DH, Price MJ. Patient and physician perspectives on the impact and management of perennial and seasonal allergic rhinitis. Clin Otolaryngol 2000;25:551-7.
2. Kosrirukvongs P, Visitsunthorn N, Vichyanond P, Bunnag C. Allergic Conjunctivitis. Asian Pac J Allergy Immunol 2001;19:4:237-44.
3. Bousquet J, van Cuawenberge P, Khaltaev N, ARIA Workshop Group. Allergic rhinitis and its impact on asthma. ARIA Workshop Report. J Allergy Clin Immunol 2001;108:5;Suppl:S147-334.
4. Illi S, von Mutius E, Lau S, et al. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol 2001;108:709-14.
5. Bousquet J, Khaltaev N, Cruz A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008;63;Suppl;86:8-160.
6. Gradman J, Wolthers O. Allergic conjunctivitis in children with asthma, rhinitis, and eczema in a secondary outpatient clinic. Pediatr Allergy Immunol 2006;17:524-6.
7. Bernstein DI, Levy AL, Hampel FC, et al. Treatment with intranasal fluticasone propionate significantly improves ocular symptoms in patients with seasonal allergic rhinitis. Clin Exp Allergy 2004;34:952-7.
8. DeWester J, Philpot EE, Westlund RE, et al. The efficacy of intranasal fluticasone propionate in the relief of ocular symptoms associated with seasonal allergic rhinitis. Allergy and Asthma Proc 2003;24:331-7.
9. Kaiser HB, Naclerio RM, Given J, et al. Fluticasone furoate nasal spray: A single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 2007;119:1430-7.
10. Fokkens WJ, Jogi R, Reinartz S, et al. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy 2007;62:9:1078-84.
11. Abelson MB, Gomes PJ, Vogelson CT, et al. Effects of a new formulation of olopatadine ophthalmic solution on nasal symptoms relative to placebo in two studies involving subjects with allergic conjunctivitis or rhinoconjunctivitis. Curr Med Res Opin 2005;21:5:683-91.
12. Bielory L. Ocular symptom reduction in patients with seasonal allergic rhinitis treated with the intranasal corticosteroid mometasone furoate. Ann Allergy Asthma Immunol 2008;100:272-9.
13. Abelson MB, Turner D. A randomized, double-blind, parallel-group comparison of olopatadine 0.1% ophthalmic solution versus placebo for controlling the signs and symptoms of seasonal allergic conjunctivitis and rhinoconjunctivitis. Clin Ther 2003;25:3:931-47.
14. Crampton HJ. Comparison of ketotifen fumarate ophthalmic solution alone, desloratadine alone, and their combination for inhibition of the signs and symptoms of seasonal allergic rhinoconjunctivitis in the conjunctival allergen challenge model: A double-masked, placebo- and active-controlled trial. Clin Ther 2003;25:7:1975-87.
15. Spangler DL, Abelson MB, Ober A, Gomes PJ. Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models. Clin Ther 2003;25:8:2245-67.
16. Chastain JB, Sindwani R. Anatomy of the orbit, lacrimal apparatus, and lateral nasal wall. Otolaryngol Clin N Am 2006;39:855-64.
17. Dowley AC, Homer JJ. The effect of inferior turbinate hypertrophy on nasal spray distribution to the middle meatus. Clin Otolaryngol 2001;26:488-90.
18. Lebel B, Bousquet J, Morel A, et al. Correlation between symptoms and the threshold for release of mediators in nasal secretions during nasal challenge with grass-pollen grains. J Allergy Clin Immunol 1988;82:869-77.
19. Zilstorff-Pederson K. Quantitative measurements of the nasolacrimal reflex. Arch Otolaryngol 1965;18:457-62.
20. Philip G, Baroody FM, Proud D, et al. The human nasal response to capsaicin. J Allergy Clin Immunol 1994;94:1035-45.
21. Montagu A. Natural selection and the origin and evolution of weeping in man. JAMA 1960;174:4:130-5.
22. Baroody FM, Foster KA, Markaryan A, et al. Nasal ocular reflexes and eye symptoms in patients with allergic rhinitis. Ann Allergy Asthma Immunol 2008;100:194-9.
23. Abelson MB, Allansmith MR, Friedlander MH. Effects of topically applied ocular decongestants and antihistamines. Am J Ophthalmol 1980;90:254.
24. Crampton HJ. A comparison of the relative clinical efficacy of a single dose of ketotifen fumarate 0.025% ophthalmic solution vs. placebo in inhibiting the signs and symptoms of allergic rhinoconjunctivitis as induced by the conjunctival allergen challenge model. Clin Ther 2002;24:11:1800-8.
25. Meltzer EO, Hampel FC, Ratner PH, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 2005;95:6:600-6.
26. Abelson MB, Welch DL. An evaluation of onset and duration of action of Patanol (olopatadine hydrochloride ophthalmic solution 0.1%) compared to Claritin (loratadine 10 mg) tablets in acute allergic conjunctivitis in the conjunctival allergen challenge model. Acta Ophthalmol Scand 2000;78:Suppl:S60-S63.
27. Chun DK, Shapiro A, Abelson MB. Ocular Pharmacokinetics. In: Albert DM, Miller JM, eds. Principles and Practice of Ophthalmology, 3rd Ed. Canada: Saunders Elsevier, 2008:179-92.
28. Lanier BQ, Abelson MB, Berger WE, et al. Comparison of the efficacy of combined fluticasone propionate and olopatadine versus combined fluticasone propionate and fexofenadine for the treatment of allergic rhinoconjunctivitis induced by conjunctival allergen challenge. Clin Ther 2002;24:7:1161-74
29. Berger W, Abelson MB, Gomes PJ, et al. Effects of adjuvant therapy with 0.1% olopatadine hydrochloride ophthalmic solution on quality of life in patients with allergic rhinitis using systemic or nasal therapy. Ann Allergy Asthma Immunol 2005;95:361-71.