Neovascular glaucoma has a relatively low prevalence, but it contributes significantly to vision loss and visual morbidity. Unfortunately, patients often don’t present with symptoms in the disease’s early stages, which makes detecting and preventing neovascular glaucoma challenging. Here, I’ll discuss neovascular glaucoma’s risk factors, the imaging modalities best suited for early disease detection, and medical and surgical management approaches.
Pathogenesis
 |
|
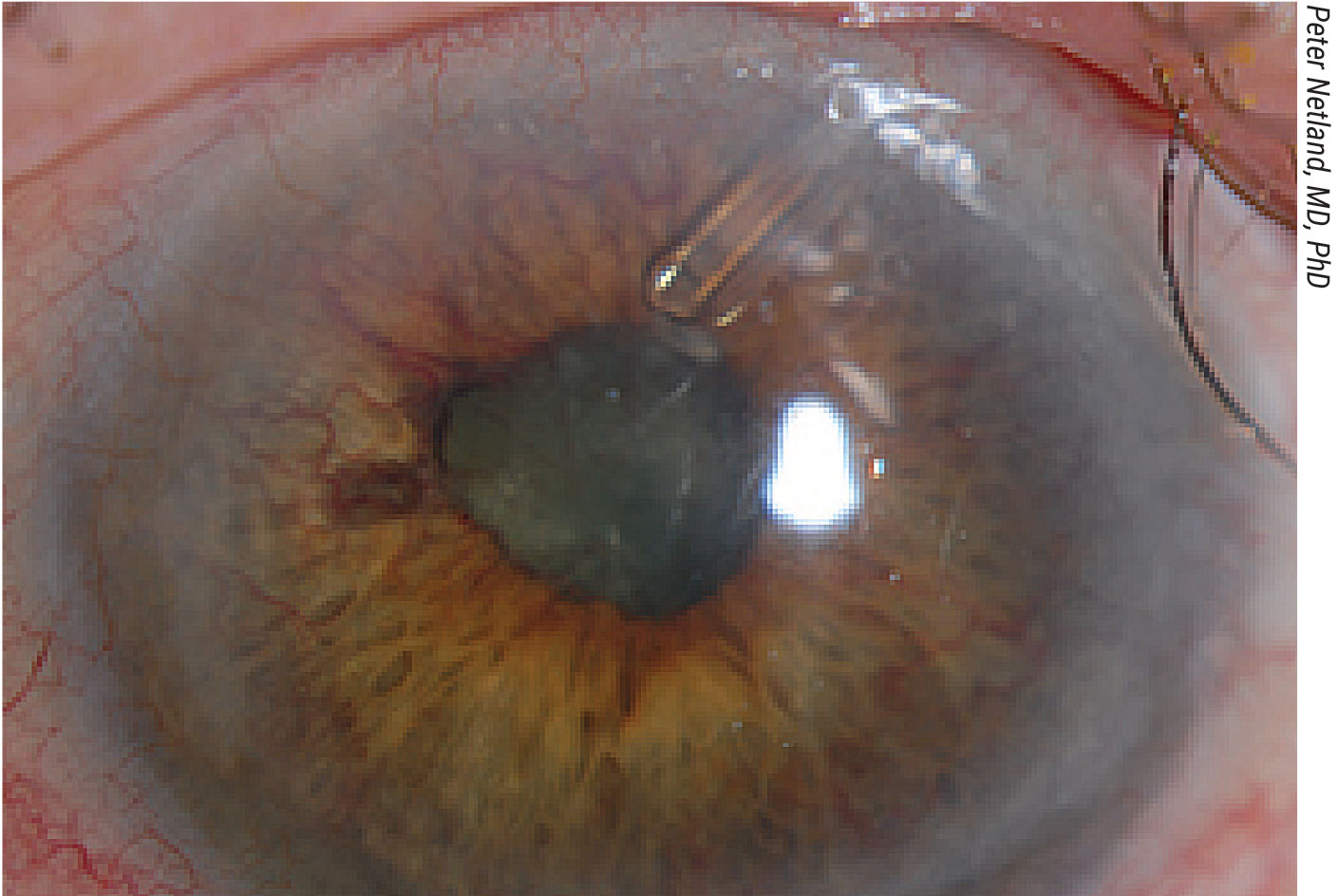
An 84-year-old patient with neovascular glaucoma after CRVO with an Ahmed valve. Ischemic CRVO is one of the most common predisposing conditions for neovascular glaucoma, responsible for about 33 percent of cases. The other two most common predisposing conditions are diabetic retinopathy (33 percent) and ocular ischemic syndrome (13 percent).11 |
Retinal ischemia is the main driving force behind the disease. The insufficient supply of oxygen causes pathogenic and angiogenic growth factors to be released into the retinal circulation and into the anterior segment. These factors cause new vessel growth and fibroblast proliferation, increased vascular permeability and leakage, endothelial cell mitosis, and leucocyte adhesion to endothelial cells with subsequent breakdown of the blood-retina-barrier.1
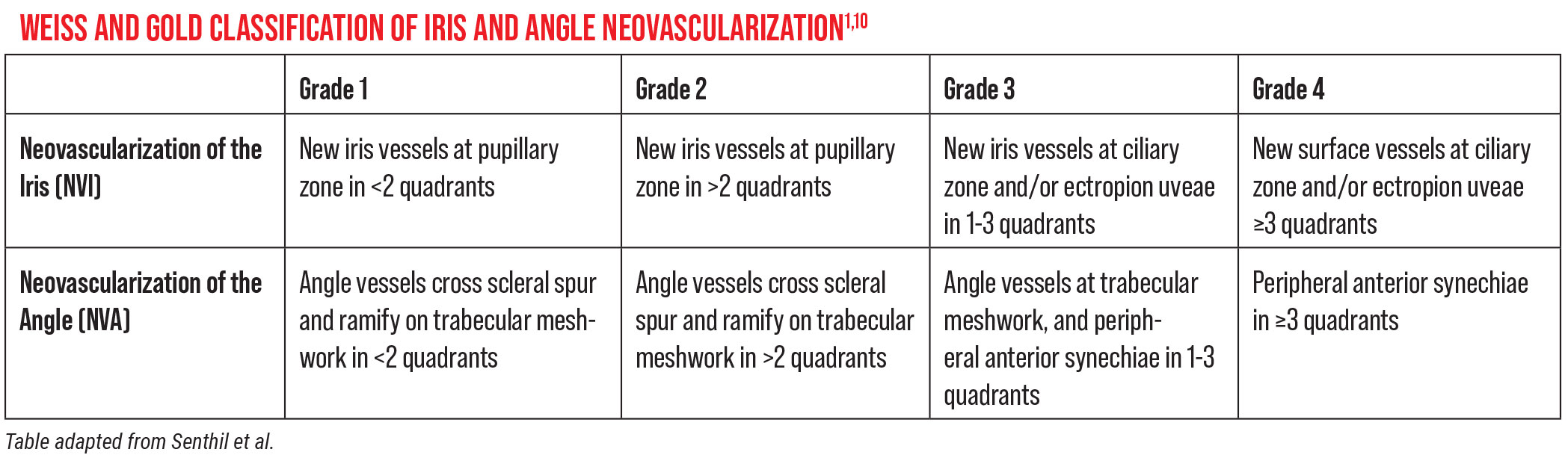
This triggers neovascularization of the iris (NVI) and the angle (NVA) (see Table below for classifications). NVI and NVA are considered very early stages of neovascular glaucoma. If these conditions aren’t caught and treated early on, then NVI and NVA can lead to trabecular meshwork obstruction and the open-angle stage, followed by proliferation of fibrous tissue that contracts and closes the anterior chamber angle over time. The angle can then become closed secondarily by peripheral anterior synechiae or scarring. All of this contributes to acute IOP elevation.
 |
Until that point, patients are generally asymptomatic. Then, patients will present suddenly with eye pain, very high pressures and the classic clinical symptoms. At this point, it’s too late to prevent, as pathogenesis has already occurred. It’s crucial to catch the disease early and treat it before it comes to this.
Anticipate
Early detection and prevention are the best medicine when it comes to neovascular glaucoma. Common causes or medical conditions that may increase your patient’s risk for developing the disease include a history of central retinal vein occlusion (especially the ischemic variant of CRVO); a history of diabetes (particularly proliferative diabetic retinopathy); and ocular ischemic syndrome. These three conditions are associated with about 75 percent of neovascular glaucoma cases. Patients who have these conditions often have a history of carotid artery occlusion, which comes from atherosclerosis, a major cause of heart disease and other cardiovascular issues in this country.
Less common causes include inflammatory conditions such as uveitis; a history of eye trauma; retinal detachment (particularly chronic retinal detachment leading to proliferative vitreoretinopathy); a history of ocular tumors such as uveal melanoma or retinoblastoma; and systemic diseases such as lupus and carotid cavernous fistula.
Prepare
If you suspect a patient may be at higher risk for neovascular glaucoma, there are a few approaches for early detection. First, conduct a thorough physical exam of the anterior chamber and its structures. Keep in mind that the angle will appear open until later stages of neovascular glaucoma, and NVA may be visible with or without NVI. Gonioscopy with a viscous coupling agent is really the only way to get a good look at the angle.
New vessels may appear as small tufts at the pupillary margin or in meandering patterns on the surface of the iris. You may see fine arborized vessels approaching the angle on gonioscopy. These will cross over, rather than behind, the scleral spur and onto the trabecular meshwork.2
If you don’t see any abnormal vessels after performing gonioscopy, but you have a high index of suspicion for neovascular glaucoma, an iris fluorescein angiogram may help you to visualize signs of the disease. (As iris fluorescein angiography is an invasive test involving the injection of a contrast dye, you may not want to perform this on everyone, but it’s an option.) Another option we have now is to use optical coherence tomography angiography to detect abnormal iris vessel growth.
For patients with cardiovascular conditions, you may also want to consider ordering a Doppler ultrasound of the carotid arteries. If your patient has significant carotid artery blockage, they may need to be treated first by a vascular surgeon who can clean out the plaque buildup. This can help prevent the potential ocular complications of neovascular glaucoma, but perhaps even more importantly, this could save the patient’s life—carotid artery stenosis increases the likelihood of stroke.
Keep in mind that in these incipient stages when you’re trying to catch neovascular glaucoma early, the patient’s intraocular pressure often won’t be elevated. You need to have a high index of suspicion, conduct a thorough exam and use some of these tests as needed to pick up early changes.
Manage the Damage
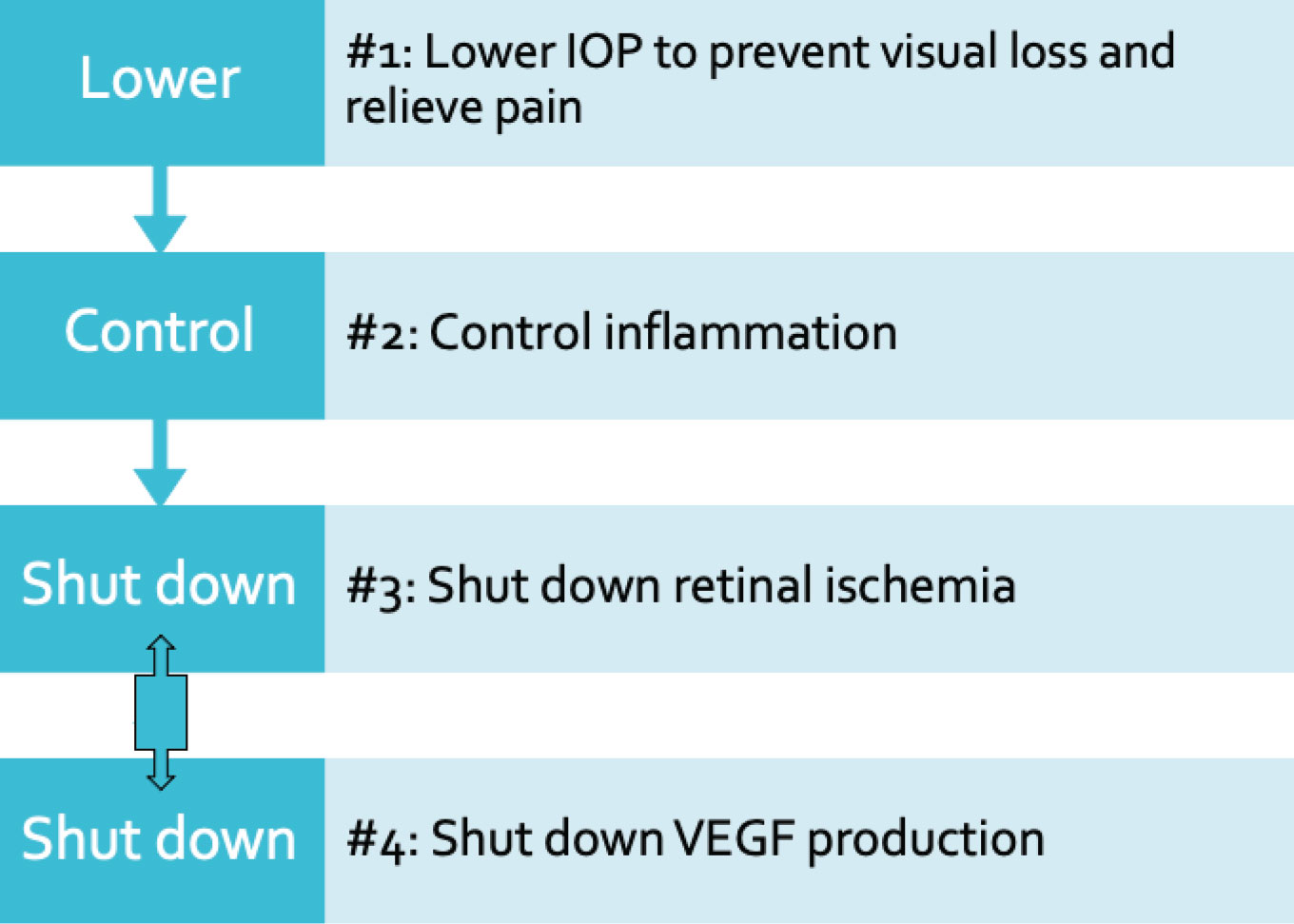
If your patient presents with high IOP and a closed angle from secondary fibrovascular membrane, the neovascular glaucoma process is in full swing. There are four key components for disease management (see flowchart):
1. Reduce the IOP to prevent visual loss and relieve the pain. Medical management will help lower IOP in the acute setting. When it comes to medical management of IOP for neovascular glaucoma, drug classes include beta adrenergic antagonists, alpha-2 agonists and topical and/or oral carbonic anhydrase inhibitors. Newer classes of medication include rho kinase inhibitors and nitric oxide component medication.
While prostaglandin analogues can be used, there are some data that suggest inflammation in the acute setting could be worsened by their use because they cause a breakdown of the blood-aqueous barrier. However, this isn’t necessarily a fully accepted argument. So, you may find that prostaglandin analogs are a useful adjunct to your medical therapy in the acute setting.
Cholinergic agents such as pilocarpine should be avoided more often than not when treating neovascular glaucoma because they can increase inflammation. They can also worsen synechial angle closure when used chronically and decrease uveoscleral outflow, which is counterproductive since increasing uveoscleral outflow is the best way to lower IOP in the acute setting. For this reason, we don’t tend to use cholinergic agents as first-line treatments.
 |
|
There are four key components to managing neovascular glaucoma. The third and fourth steps, shutting down the inciting process (retinal ischemia) and VEGF production, should be undertaken simultaneously. This is typically done using PRP and anti-VEGF injections, respectively. |
2. Lower the inflammation in concert with IOP reduction. Eyes affected by neovascular glaucoma tend to become very inflamed, so as you bring the pressure down, take steps to reduce the inflammation as well. We generally use topical corticosteroids. Using a cycloplegic such as atropine can also help calm the eye and decrease inflammatory processes. This will also help to relieve the pain.
3. Shut down the inciting process. Addressing the inciting process, the retinal ischemia, and the fourth component, targeting VEGF production, should be undertaken simultaneously. With the help of our retina colleagues, we shut down the retinal ischemia by performing retinal ablation. Removing the ischemic retinal tissue will secondarily reduce the production of angiogenic factors such as VEGF.
Panretinal photocoagulation is the mainstay of therapy. It’s usually performed under topical anesthesia over one to three sessions using a slit lamp or indirect laser with 1,200 to 1,600 burns using an approximately 500-µm spot size.3 PRP isn’t always possible to perform right away, however. It may be difficult to obtain good visualization of the retina in the acute setting of neovascular glaucoma because the eye is often cloudy and edematous from the high IOP. In these cases, try to lower the pressure to clear the cornea. Once you have a better view, you can reattempt PRP until the neovascularization resolves. Keep in mind that NVI and/or NVA may not regress until four to six weeks after PRP.
PRP for ocular ischemic syndrome is indicated for posterior and anterior segment neovascularization. However, PRP alone may compromise the optic nerve head blood flow. Be sure to work with your vascular surgery colleagues or cardiologists to improve retinal blood flow with carotid endarterectomy or other interventions when trying to shut down these abnormal vessels. And always keep your patient’s particular medical conditions in mind when instituting these treatments.
4. Target VEGF production as well. Anti-VEGF injections can cause regression of the abnormal blood vessels in neovascular glaucoma. However, this is a temporary regression, lasting about four to six weeks, and efficacy depends on the stage of the neovascular glaucoma you’re treating.
If the disease is very advanced, studies indicate that you may not get much benefit from using anti-VEGF injections. A Cochrane systematic review of all the available data reported that long-term outcomes in neovascular glaucoma don’t seem to differ with or without the use of anti-VEGF agents.4 Notably, there were only four randomized clinical trials in the review with substantial heterogeneity of methods and analysis (in China, Brazil, Egypt and Japan). So again, these agents are more of an acute temporizing measure to attempt to shrink the blood vessels, often in preparation for a more definitive surgical management approach, which will be needed to fully control the IOP.
Surgical Management
Surgical options include trabeculectomy with mitomycin-C, glaucoma drainage devices, cyclophotocoagulation or endocyclophotocoagulation and combined procedures. There are several different glaucoma drainage device options, all of which have pros and cons. Much of the decision will be based on surgeon preference. CPC and ECP have IOP-lowering effects similar to those of a glaucoma drainage device, but there are potentially more complications with them.5 Combining a glaucoma drainage device with a pars plana vitrectomy is another option with good IOP lowering potential. It may help long-term management of retinal ischemia and can be a good option if there’s concomitant retinal pathology such as vitreous hemorrhage; however, there’s also a potential for increased vision loss with these procedures.
There’s some published data suggesting that intravitreal anti-VEGF is associated with greater overall success rates with Ahmed glaucoma valve implantation when administered preoperatively.6 The rationale is that if you can manage the potential for bleeding intraoperatively and postoperatively that will lead to higher success rates and less chance for intra- and postoperative complications such as hyphema.
A retrospective study by Marwan Sahyoun, MD, and colleagues reported that while bevacizumab given seven days prior to surgery wasn’t associated with better surgical success, BCVA or IOP control, its administration significantly decreased postoperative hyphema and the number of glaucoma medications patients used at the final visit.7 Similarly, a prospective study in which patients were given intravitreal ranibizumab one week before trabeculectomy reported significantly decreased IOP, a significant but modest visual acuity improvement and fewer postoperative complications than Ahmed valve implantation alone.8
The other option is to administer anti-VEGF agents intraoperatively during glaucoma drainage device implantation, though few large studies have been conducted on this. In a randomized trial by Enyr S. Arcieri, MD, and colleagues, the treatment group received intravitreal bevacizumab injections intraoperatively as well as at four and eight weeks after Ahmed valve implantation.9 Their two-year results suggest that adjunctive use of bevacizumab intraoperatively may lower IOP and the number of glaucoma medications needed by patients postoperatively. However, this trend doesn’t appear to be statistically significant.3
Prognosis
Unfortunately, once neovascular glaucoma presents, it’s often in the end-stages of the disease process. In general, the prognosis is guarded. Much depends on when you’re able to intervene. We see more extensive damage in patients who don’t come in right away, for whatever reason, and that can affect the outcome. Situations in which there’s high IOP and abnormal blood vessel proliferation are very time-sensitive when it comes to avoiding permanent damage to the retina and the eye’s drainage system. I tell my patients that this is a very serious condition and that there’s a relatively high chance they’ll lose vision permanently.
To sum up, neovascular glaucoma is a serious, sight-threatening condition characterized by acute IOP increase and abnormal blood vessel growth in the iris and angle. Treatment consists mainly of prevention through early detection, as well as urgent management, ultimately leading to surgical intervention. Prognosis depends heavily on when intervention begins, but it remains poor.
As our population continues to suffer from increased incidence and prevalence of diabetes and other vasculopathic conditions, we need to keep in mind that members of this ever-growing patient population could fall prey to neovascular glaucoma. We should strive to gather stronger data using larger sample sizes and adopt a core outcome set so data from various studies can be combined in meta-analyses. We must also attempt to stratify randomization in trials based on etiology to help us better understand treatment effectiveness for neovascular glaucoma.
Dr. Syed is a professor of ophthalmology and glaucoma specialist. She also serves as the assistant dean for educational affairs at the John Sealy School of Medicine, and vice chair for academic affairs in the Department of Ophthalmology at the University of Texas Medical Branch.
1. Senthil S, Dada T, Das T, et al. Neovascular glaucoma – A review. Indian J Ophthalmol 2021;69:3:525-534.
2. Chandler PA, Grant WM. Lectures on glaucoma, Philadelphia, 1965, Lea and Febiger.
3. Rodrigues GB, Abe RY, Zangalli C, et al. Neovascular glaucoma: A review. Int J Ret Vit 2016:2:26.
4. Simha A, Aziz K, Braganza A, et al. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database of Systematic Reviews 2020;2:CD007920.
5. Shchomak Z, Sousa DC, Leal I, et al. Surgical treatment of neovascular glaucoma: A systematic review and meta-analysis. Graefe’s Arch Clin Exp Ophthalmol 2019;257:1079-1089.
6. Zhou M, Xu X, Zhang X, Sun X. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: A systematic review and meta-analysis. J Glaucoma 2016;25:7:551–557.
7. Sahyoun M, Azar G, Khoueir Z, et al. Long-term results of Ahmed glaucoma valve in association with intravitreal bevacizumab in neovascular glaucoma. J Glaucoma 2015;24:5:383–388.
8. Liu L, Xu Y, Huang Z, Wang X. Intravitreal ranibizumab injection combined with trabeculectomy versus Ahmed valve surgery in the treatment of neovascular glaucoma: Assessment of efficacy and complications. BMC Ophthalmol 2016;16:65.
9. Arcieri ES, Paula JS, Jorge R, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2-year follow-up. Acta Ophthalmol 2015;93:1:e1-6.
10. Weiss D, Gold D. Neofibrovascularization of iris and anterior chamber angle: A clinical classification. Ann Ophthalmol. 1978;10: 488–91.
11. Havens SJ, Gulati V. Neovascular glaucoma. Dev Ophthalmol 2016;55:196-204. [Epub October 26, 2015].



