For those of us treating glaucoma, these are exciting times. The last new class of glaucoma medications—the prostaglandin analogues—be-came available back in 1996. Now, more than 20 years later, we find ourselves able to prescribe two new drugs that represent entirely new classes of glaucoma medication. Vyzulta (latanoprostene bunod oph-thalmic solution 0.024%, from Bausch + Lomb) and Rhopressa (netarsudil ophthalmic solution 0.02%, from Aerie Pharmaceuticals) each have mechanisms of action different from those of the drugs that were previously available. Both of these are promising and should serve as important primary or adjunctive glaucoma therapies.
Today, most ophthalmologists’ glau-coma treatment paradigm starts with prescribing a prostaglandin analogue as first-line therapy. That’s true for three main reasons: Prostaglandins are currently the most effective class of IOP-lowering drug; they’re safe in terms of their side-effect profile; and they’re used once daily, which minimizes the impact on patients’ quality of life and maximizes ad-herence. Both of the new drugs do well in the same three categories: Both are effective (though not equally effective) IOP-reducers; both have favorable systemic side-effect profiles; and both are once-a-day medications.
Here, I’d like to discuss the benefits, limitations and potential drawbacks of each medication and offer some thoughts on how they might fit into our treatment paradigm.
Latanoprost Redux
Latanoprostene bunod (Vyzulta) is a molecule that is metabolized into latanoprost, which we’ve had many years of experience with, and a moiety that donates nitric oxide once the medication is in the eye. Latanoprost works primarily by increasing uveo-scleral outflow, while nitric oxide in-creases trabecular outflow, which is a novel mechanism of action. Because of these complementary mechanisms, combining the two molecules offers favorable IOP reduction.
The data from the clinical trials shows robust efficacy for latanopros-tene bunod. The reduction in mean diurnal pressure at about a month was almost 9 mmHg.1 For a single agent used once a day to deliver 9 mmHg IOP reduction at peak effectiveness is impressive. Also, at that time point, latanoprostene bunod was superior to latanoprost alone, and the difference was statistically significant.
In terms of the side-effect profile, studies have found that Vyzulta is safe systemically. That’s very favorable for glaucoma patients, who are often on other systemic medications to treat cardiovascular or pulmonary diseases. The most common ocular side effect with Vyzulta was hypere-mia, which is not unexpected since this is a prostaglandin. There were other adverse effects related to topical instillation, such as irritation and dry eye, which are not unique to latanoprostene bunod and are encountered with other glaucoma topical therapies. Overall, discon-tinuation due to adverse effects has been rare with latanoprostene bunod in published studies.2
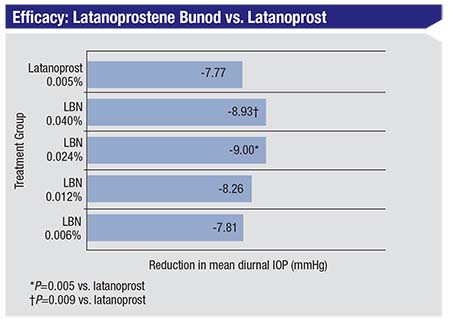 |
| In the VOYAGER trial, latanoprostene bunod 0.024% produced a reduction in mean diurnal IOP of 9.0 mmHg. LBN was also significantly superior to latanoprost alone at peak effect.1 |
Of course, one advantage of latano-prostene bunod is that we have many years of experience with latanoprost, a key component of the new drug. As a result, we’re well acquainted with its adverse effects. That gives us a leg up in terms of knowing what we’re dealing with.
The Dawn of ROCK-inhibitors
What’s most exciting about netarsudil (Rhopressa) is that it’s the first in a new class of glaucoma medi-cations. Unlike the other classes of medications available to us, rho kinase inhibitors have a different mechanism of action: They work by enhancing trabecular meshwork outflow. In contrast, prostaglandins in-crease uveoscleral outflow, while other medications, such as alpha agonists, topical carbonic anhydrase inhibitors and beta blockers mainly decrease aqueous production. Combining some of the current medications with similar mechanisms of action simply maximizes that effect, and the IOP reductions may not be additive. Because rho kinase inhibitors affect trabecular meshwork outflow, netarsudil may combine well synergistically with some of the other currently available medications. As adjunctive therapy, that’s very favorable.
In terms of efficacy, netarsudil didn’t initially meet noninferiority criteria vs. timolol in the ROCKET-1 study, in which baseline washout IOP was between 20 and 27 mmHg. However, a post-hoc analysis revealed that netarsudil was non-inferior to timolol for patients with a post-washout IOP lower than 25 mmHg. The most recent data is from the ROCKET-4 trial, with a six-month extension study. This was presented at the 2018 meeting of the American Glaucoma Society. In that trial, once-daily netarsudil was compared to timolol b.i.d. in patients with primary open-angle glaucoma and ocular hypertension. Basically, the study showed that netarsudil was not inferior to timolol at all time points from week two to month six.
Overall, in the ROCKET series of trials, including ROCKET-4, netarsudil q.d. was noninferior to timolol (dosed twice daily) at a range of baseline IOPs. To restate that from a clinician’s standpoint, reductions in IOP were similar to timolol. We know that timolol generally delivers a 20- to 25-percent pressure reduction, depending on how high the baseline pressure is and how responsive the patient is to beta blockers. We expect netarsudil to fall within that range.
In terms of adverse effects, like latanoprostene bunod, netarsudil is a patient-friendly medication that’s used once a day and has no cardiac or pulmonary impact; the most common adverse effect is conjunctival hyper-emia. In the ROCKET trials, about half the patients using once-a-day netarsudil reported hyperemia. This is a higher rate than we’ve seen with beta blockers. However, the hyperemia was not present at every visit; it was intermittent and for the most part was mild and transient and reported by the investigator rather than the patient.3
Rho kinase inhibitors do have two unique adverse effects that we’ve learned of from the clinical trials that are not typical of other currently used glaucoma medications. One is conjunctival hemorrhages. Interestingly, these were very small—typically described as “pinpoint hemorrhages.” In fact, they were so small that they were usually only observed by the investigators; most patients didn’t notice them. They were considered mild in the vast majority of cases, and as you might expect, they resolved on their own. They were not seen as a justification for discontinuation of the medication.
The other unique adverse effect that’s been observed with netarsudil—and rho kinase inhibitors in general—is corneal verticillata (5 to 9 percent in ROCKET-1 and -2). This is a benign condition in which lipid deposits form in the corneal epithelium. This phenomenon was graded as mild in most patients. In fact, the verticillata were not symptomatic; because they occur in the superficial layers of the corneal epithelium, they tend to not be visually significant. Patients didn’t notice any changes in their vision, so this phenomenon was only noted by the investigators. Furthermore, the verticillata resolved once the medication was discontinued. (Of note, other FDA-approved medications can cause verticillata; amiodarone is the classic example. However, this may be the first ex-ample of verticillata being caused by a topical glaucoma medication.)
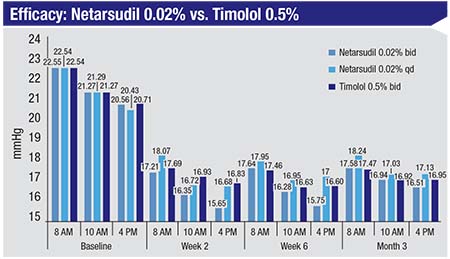 |
| Netarsudil 0.02% used once a day in the ROCKET-2 trial was not inferior to timolol 0.5% dosed twice a day up to three months.3 |
It’s important to keep in mind that all of this data regarding latanoprostene bunod and netarsudil comes from clinical trials, not from clinical use in the field. Until both medications are widely used after their launch, we won’t be able to say definitively how significant or insignificant these findings may be.
First-line or Adjunct?
The way in which these two novel medications will end up being used is difficult to predict at this point, but their relative efficacy may be a significant factor. For example, latanoprostene bunod is one of the most effective medications we currently have, in addition to being used once a day. This will position it well as a first-line medication when you’re trying to achieve significant (on the order of 30 percent or greater) reduction in pressure to get to a target at which you can stabilize the disease. It also makes sense for patients in terms of avoiding having to dose medications multiple times a day, and for patients who have cardiovascular disease, where you’re trying to avoid medications that can interact with systemic illnesses and medications. Most patients are on prostaglandins as first-line therapy, and latanoprostene bunod is in line with that.
The challenge with latanoprostene bunod, as with many medications that are newly released, is commercial insurance coverage. When a medi-cation is first released, it’s often not covered by a lot of plans. Until that situation changes, it may limit the access many patients have to latanoprostene bunod and netarsudil. Of course, post-release, the situation with coverage is dynamic and is likely to improve or change over time.
Netarsudil, which causes IOP re-ductions closer to timolol, would make total sense as an adjunctive therapy. I’d say about half of our glaucoma patients require adjunctive therapy to get to their target or goal pressures. Currently we have a choice of several medications as adjunctive treatments, but all of them have to be used two or three times daily. (Beta blockers can be used once a day, but they have systemic side effects.) So netarsudil is well-positioned to be a choice adjunctive agent.
There will definitely be some patients for whom prostaglandin analogues are not ideal choices for first-line treatment for a number of possible reasons. For example, this would be the case for patients with active uveitis and patients who are concerned about iris color changes. I have a few patients in my own practice who have mixed iris colors, and they don’t want to risk changing this, so they refuse the medication. Also, prostaglandins can cause periorbital fat atrophy. Some patients see the appearance of their upper eyelids change over time, and you end up having to take them off of the drug. (Subtle changes may be noticed more in patients who are only being treated in one eye.) In our practice, we currently offer these patients laser trabeculoplasty or one of the other adjunctive therapies, all of which have to be used twice a day or more. Netarsudil is a possible choice for those patients because it’s once a day, which is a big advantage.
One obvious question is whether the two new medications might be complementary. Both medications have an effect on outflow through the trabecular meshwork, but we currently have no data on how they’ll interact. We do know that their mechanisms of action are not the same, just as many drugs that reduce aqueous humor production use different mechanisms of action. It’s possible that either of them alone might optimize trabecular meshwork outflow, but it’s also possible that their interaction could be synergistic. It will probably require post-launch studies and clinical experience over time to answer that question.
What Lies Ahead?
Given that these medications just became available, I think it’s too soon to guess how clinicians and patients will interact with them. I can definitely vouch for the level of excitement about these new medications, as many glaucoma patients in my practice have inquired about them. Currently, we have data from the clinical trials, which was certainly favorable for both of these drugs. But what I’ve learned from my own experience is that until we get our hands on them and use them clinically, their full potential will remain unknown, and many questions about their side effects will remain unanswered.
The reality is that clinical trial data doesn’t always match what we find in clinical practice. For example, historically there were three very large clinical trials of latanoprost in the United States and abroad. We learned a lot about the side effects and the efficacy profile of the drug from those trials, but we completely missed the side effect of eyelash growth. That was only picked up later on after the medication was widely used.
The point to make is that it can be hard to translate clinical trial data into real-world data. We’ll need to observe the drugs’ real-world efficacy, how our patients react to them, what kind of side-effect profiles we see in day-to-day use and how each drug combines with other medications, which is a key aspect of treating glaucoma. Clinical trials give us baseline information and clean data on the efficacy of the medications and their safety, but it remains to be seen how the drugs will perform once widely launched and used. Our experience could very well mirror what was discovered during the clinical trials, or it could be different. That’s something we will learn in the months to come once we start using these two drugs. REVIEW
Dr. Khouri is an associate professor of ophthalmology, as well as director of the glaucoma division and the residency program, at Rutgers New Jersey Medical School. He has received grant support from Allergan, Bausch + Lomb and Aerie Pharmaceuticals, is a consultant for Aerie, and is on the speaker bureau for Bausch + Lomb, Allergan and Novartis.
1. Weinreb RN, Ong T, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol 2015;99:6:738-45.
2. Medeiros FA, Martin KR, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: The LUNAR study. Am J Ophthalmol 2016;168:250-259.
3. Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol 2018;186:116-127.