Optogenetics is a modern technology that is just beginning to see real-world applications in health care. In ophthalmology, optogenetics can play a role in treating various diseases, from common cases to rare forms of vision loss. This article will examine the unique treatment and how it’s being applied to ophthalmology today.
What is Optogenetics?
In 2005, Karl Deisseroth, MD, PhD, a professor at Stanford University, published his paper on optogenetics. Along with MIT professor Ed Boyden, PhD, they pioneered a novel concept to target and control cells in the biological systems of animals. Optogenetics is the combination of genetic and optical methods to achieve gain or loss of function of well-defined events in specific cells of living tissue.1 Basically, optogenetics uses light-sensitive technology in the form of opsin proteins to deliver light into tissues, target the control tools to cells of interest and obtain compatible readouts and analyses,1 allowing for diagnostic implications.
In ophthalmology, optogenetics aims to restore vision by replacing lost or dysfunctional photoreceptors by inserting opsins, or light-sensitive proteins, into the downstream retinal neurons that have no intrinsic light sensitivity.2 Opsin genes are divided into two groups: microbial opsins and animal opsins. Microbial opsins derived from algae and other microorganisms are more widely used as optogenetic tools in neuroscience in order to impart light-induced membrane permeability to neurons.2
“There are more simple systems that you find mainly in microbial organisms,” says Joseph N. Martel, MD, an assistant professor of ophthalmology at the University of Pittsburgh School of Medicine. “These are very rudimentary simple systems. Usually, light-gated ion channels that change the polarization of the cells. Then, there are more sophisticated systems in the mammalian system. For example, light sensitive G protein-coupled receptors that when activated by light, they initiate an intercellular cascade of signals that then change the intercellular dynamics and activate or deactivate the cell depending on the type of channel, or the type of opsin.”
Channelrhodopsins (ChR), which are isolated from the green algae Chlamydomonas, were the first opsins to be identified as optogenetic tools.2 Other opsin classes that have been used in ophthalmology treatments include halorhodopsin (HR), melanopsin (OPN4) and human rhodopsin (RHO).
For optogenetic vision restoration, adeno-associated virus vectors are used to deliver opsin proteins into the retinal cells. AAV is a non-enveloped virus that can be engineered to deliver DNA to target cells.3 Optogenetic treatments in the current FDA pipeline use intravitreal injections to administer AAV vectors, but studies have shown that subretinal injections are also a viable option.2
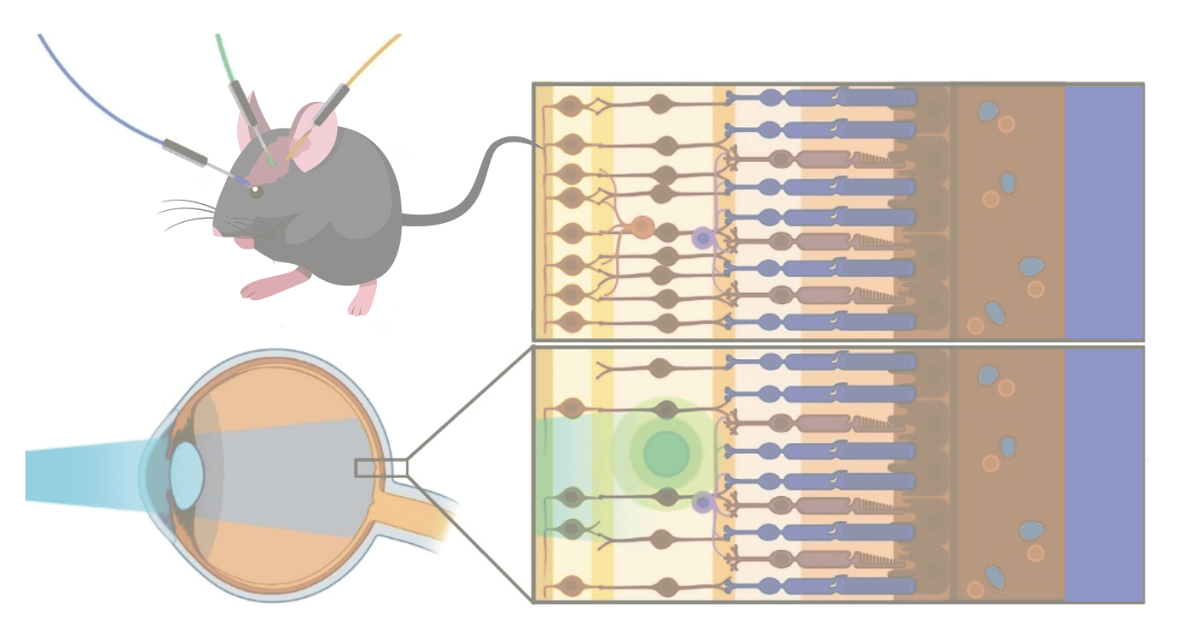 |
| This mouse model represents how different opsin proteins are activated by specific wavelengths of light illumination. In this case, the blue light is targeting and activating the optogenetic molecules represented by the green indicator, but not the molecules represented by yellow indicator. Therefore, the blue light is at the appropriate wavelength for the green indicator and not the yellow indicator. Adapted from “Prosseda PP, Tran M, Kowal T, et al. Advances in ophthalmic optogenetics: Approaches and applications. Biomolecules 2022;8;12:2:269” under the terms of https://creativecommons.org/licenses/by/4.0/#. |
Depending on the optogenetic therapy, the opsins used will function differently in the retina. “Most of them target the retinal ganglion cells, but the multi-characteristic opsin, the MCO-010, targets the bipolar cells,” says Dr. Martel. Furthermore, different opsin proteins will have different thresholds of light sensitivity. “There are other trials, like the GenSight PIONEER trial, that uses CrimsonR, which is a very simple opsin. That particular protein is only sensitive to a very narrow band of wavelength of light, which I think is around 580 to 600 nm,” he continues. In this case, pharmaceutical companies, like GenSight, have developed medical devices to assist with vision restoration by adjusting light wavelengths.
Optogenetic therapy is currently being developed in ophthalmology for diseases leading to severe vision loss. The diseases at the core of optogenetic pharmaceuticals are retinitis pigmentosa, choroideremia and Stargardt’s disease. Retinitis pigmentosa is a group of disorders that produce a gradual loss of vision, and it affects one in 5,000 people worldwide.4 Choroideremia leads to the degeneration of the retinal pigment epithelium, photoreceptors and choriocapillaris, and it affects between one in 50,000 to one in 100,000 people worldwide.5 Lastly, Stargardt’s disease is the most common cause of juvenile macular dystrophy and it leads to the accumulation of lipofuscin in the retina.6 It affects 10 to 12.5 per 100,000 people in the United States.6
“I think the last couple years have demonstrated an important milestone in establishing the proof of principle of optogenetics—of therapeutic optogenetics—for vision restoration, but there’s still more that needs to be done,” says Dr. Martel. “There are different diseases that play a role here and there’s some phenotypic variation in which cellular components of the retina degenerate and which are relatively preserved. So, there may be certain opsins that are more advantageous. We need to fine tune which opsins are best under which circumstances.”
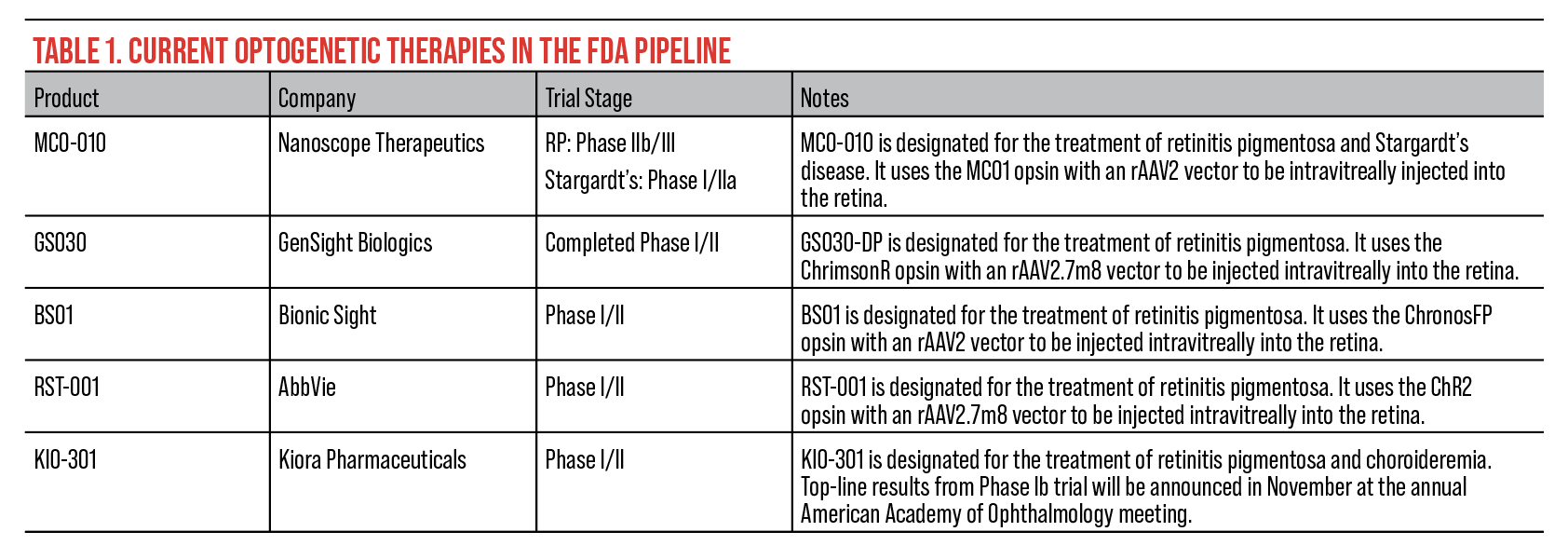 |
| Click table to enlarge. |
Current Pipeline
The current FDA pipeline for optogenetic therapy features five novel treatments. These treatments are still undergoing trials for approval.
• MCO-010 (Nanoscope Therapeutics). According to Nanoscope, MCO-010 is being clinically developed to treat retinitis pigmentosa and Stargardt’s disease. The optogenetic therapy has been granted Orphan Drug and Fast Track designation by the FDA for both diseases. This treatment is injected into the retina combining a multi-characteristic opsin protein with an AAV vector system to photosensitize bipolar cells allowing the retina to sense light again.
Currently, MCO-010 is undergoing two separate clinical trials, STARLIGHT and RESTORE, to find the safety and efficacy of treatment for retinitis pigmentosa and Stargardt’s disease. Earlier this year, Nanoscope presented its key results from the latest Phase IIb RESTORE trial of MCO-010 for the treatment of retinitis pigmentosa. In the trial, 18 patients received a single injection of MCO-010 to treat severe vision loss due to RP. Nine other patients were administered a sham intravitreal injection.
Over a one-year time period, researchers measured efficacy using Multi-Luminance Y-Mobility Tests (MLYMT), Multi-Luminance Shape Discrimination Tests (MLSDT), and Best-Corrected Visual Acuity scores. According to the RESTORE trial, a two or more luminance level change for the MLYMT or MLSDT is considered clinically meaningful, and a 0.3 logMar change in BCVA is also considered clinically meaningful. For reference, MLYMT is a vision-guided mobility test and MLSDT is a near object recognition test.
RESTORE trial researchers reported that all MCO-010 patients showed vision improvement in the MLYMT, MLSDT or BCVA compared to five patients in the placebo group. Seventeen of the MCO-010 patients showed vision improvement in the MLYMT or BCVA compared to four patients in the placebo group. Also, 16 MCO-010 patients demonstrated a two or more luminance level improvement in the MLYMT or MLSDT compared to four patients in the placebo group. Researchers noted that these results are consistent with those observed in an earlier Phase I/II trial in which nine MCO-010 patients demonstrated clinically meaningful data in BCVA and the MLYMT or MLSDT measurements.
Recently, Nanoscope presented key results from its Phase II STARLIGHT trial of MCO-010 for the treatment of Stargardt’s disease. This trial only recruited six patients with Stargardt’s. Researchers measured systemic adverse events along with BCVA scores, MLYMT, MLSDT, and sensitivity measured by Octopus Visual Field Perimetry, which measures patient reaction time. During the trial, patients experienced clinically meaningful improvements in BCVA, they exhibited an approximately 3-dB gain in mean sensitivity, and high baseline MLYMT and MLSDT performances were maintained throughout the study.
Overall, Nanoscope reported that MCO-010 is well tolerated with no severe adverse events when treating retinitis pigmentosa or Stargardt’s. Only one severe adverse event was reported in the RESTORE trial from the placebo group.
 |
|
A prototype design of GenSight Biologics’ GS030-MD goggles. According to GenSight, the front of the product features a camera which captures the natural environment and sends a stream of images to a projector inside the device. A cable runs down the side of the device and connects to a processing unit with software that alters the images to the correct light intensity. |
• GS030 (GenSight Biologics). GS030-DP is being developed for the treatment of retinitis pigmentosa. The one-year results from the PIONEER Phase I/II clinical trial of GS030-DP were announced earlier in 2023. Nine patients were administered the optogenetic therapy with a follow-up of up to four years. According to GenSight, patients were divided into three cohorts, each receiving a different dose of GS030-DP (5e10 vg; 1.5e11 vg; 5e11 vg) via an intravitreal injection in the worst affected eye. A Data Safety Monitoring Board reviewed the safety data of all the treated patients and based on their findings, recommended selecting the highest dose (5e11 vg) for the expansion cohort in future trials.
Researchers for the PIONEER trial measured the safety and tolerability of the first three completed cohorts. The patients in each cohort reported only mild and moderate ocular adverse events where the most common adverse event was mild intraocular inflammation responses to corticosteroid treatment. According to the trial report, 70 percent of patients experienced inflammation.
“GS030 uses the CrimsonR opsin protein, which requires a higher stimulation threshold than you would find in the ambient light environment,” says Dr. Martel. “There has to be a certain intensity of light.” GenSight’s treatment currently requires GS030-MD, a wearable device, designed as goggles, to assist with stimulating retinal cells.
“These goggles have a camera embedded in them that takes pictures of the visual environment,” explains Dr. Martel. “Those pictures are then processed by various AI algorithms that are then translated into a beam of light within the goggles, which is projected through the pupil and onto the surface of the retina after the retina has been treated with the optogenetic therapy.
“That light is pulsed light and it’s at the appropriate wavelength and intensity to activate the genetically modified retina that’s expressing the photosensitive proteins in the retinal ganglion cells,” continues Dr. Martel.
• BS01 (Bionic Sight). Since March 2020, 12 patients have been participating in the Phase I/II clinical trial for BS01, an optogenetic therapy for the treatment of retinitis pigmentosa. One year after the trial’s initial start date, Bionic Sight announced that the first four patients who received BS01 adapted to see light and motion, and, in two cases, developed the ability to detect the direction of motion. Additionally, two patients who received lower doses showed more than a twentyfold increase in light sensitivity compared to their baseline sensitivity, while the other two received a dose three times higher and showed more than a hundredfold increase.
In April 2023, Bionic Sight released an update on the BS01 clinical trials stating that those who’ve received the highest dose of the therapy had the most vision restored. Patients have been given vision tests throughout the trial, especially after the four top responders gained the ability to identify shapes and objects. Bionic Sight reported that the success rate for identifying objects ranged from 80 to 100 percent for the top responders. Before treatment, patients’ success rate was 25 percent when given four choices and 12.5 percent when given eight choices.
BS01 uses a ChronosFP opsin protein with an AAV vector to photosensitize the retinal ganglion cells. Similar to other optogenetic therapies, BS01 requires a device to be worn like a pair of glasses to properly transmit light to the retina. Bionic Sight states that its unique device produces neural impulses, similar to those produced by ganglion cells in a healthy retina, instead of enhancing the shape and intensity of the image.
• KIO-301 (Kiora Pharmaceuticals). Recently, Kiora announced the completion of the final patient’s last endpoint from the ABACUS trial of KIO-301. Topline study results from the Phase Ib clinical trial will be reported in November at the annual American Academy of Ophthalmology meeting.
Prior to the trial, a total of six patients were enrolled, all with late-stage retinitis pigmentosa. Earlier this year, Kiora presented preliminary results from the ABACUS trial. They presented the results from a patient with no light perception and received a low dose injection of KIO-301 in one eye. The patient reported an improvement in the ability to perceive contrast between light and dark at days seven, 14 and 29 of the trial. They also reported an improvement in object identification and a positive impact towards their overall functional vision during everyday activities. Also, it was noted that the patient’s initial dose was safe and well tolerated at day 29 without adverse events.
In addition to retinitis pigmentosa, KIO-301 is being considered for the treatment of choroideremia and other rare inherited retinal diseases.
• RST-001 (AbbVie). According to AbbVie, RST-001 was given Orphan Drug designation in 2014 and began Phase I/II clinical trials for the treatment of retinitis pigmentosa in 2015. This is an ongoing study and it’s estimated to complete in September 2024. During the study, 14 participants will be measured, each over a six-month time frame. The primary outcome is measured by the number of participants with any Grade 3 or greater adverse event related to RST-001.
AbbVie says that the study is composed of two parts. The first part is to determine the expansion cohort for future studies. The second part of the study will focus on obtaining additional safety data at the highest tolerated doses as well as provide additional clinical data to aid in the design of future studies.
Other Applications
Although the developing optogenetic therapies are focused on severe forms of vision degradation, there are other applications in ophthalmology. For instance, researchers at Stanford University studied optogenetics as a mechanism to regulate intraocular pressure.
“It starts with a clinical question: What controls pressure in the eye? That’s when we discovered this specialized structure called primary cilia,” says Yang Sun, MD, PhD, a researcher and professor of ophthalmology at Stanford University School of Medicine. “In fact, primary cilia have actually been observed in the body for almost 40-plus years. The initial observation of the cilia in the eye was made in 1976, and then subsequently in 1982 and the 2000s. They were observed in the trabecular meshwork and Schlemm’s canal,” he continues. Primary cilia can sense aqueous humor flow, therefore this plays an important role in regulating IOP.7
“The idea behind the study was to use this optogenetic technique to regulate fluid flow in different parts in the eye,” says Dr. Sun. The researchers’ study used two modified plant proteins, cryptochrome 2 (CRY2) and CIBN, introduced to inositol polyphosphate 5-phosohatase (OCRL) to modulate IOP.8 Direct exposure to blue light at a wavelength of 450 nm activated the dimerization of CRY2 and CIBN.8 Since OCRL is located in the primary cilia and plasma membrane of the trabecular meshwork cells, the activated proteins target and modulate OCRL to increase outflow facility, which correlates with a decrease in IOP.2
Optogenetics continues to advance in health care and the future looks bright for the novel technology. “I think a lot of novel therapeutic vehicles may arise from this group of gene therapy approaches,” says Dr. Sun. “Optogenetics certainly has great promise, and it will be fantastic to see it in clinical practice.”
Dr. Martel was principal investigator for GenSight Biologics’ PIONEER trial. Dr. Sun has no financial interests to disclose.
1. Deisseroth, K. Optogenetics. Nat Methods 2011;8:26-29.
2. Prosseda PP, Tran M, Kowal T, et al. Advances in ophthalmic optogenetics: Approaches and applications. Biomolecules 2022;8;12:2:269.
3. Naso MF, Tomkowicz B, Perry WL 3rd, et al. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017;31:4:317-334.
4. O’Neal TB, Luther EE. Retinitis Pigmentosa. StatPearls 2023. https://www.ncbi.nlm.nih.gov/books/NBK519518/.
5. Khan KN, Islam F, Moore AT, et al. Clinical and genetic features of choroideremia in childhood. Ophthalmology 2016;123:10:2158-65.
6. Kohli P, Kaur K. Stargardt disease. StatPearls 2023. https://www.ncbi.nlm.nih.gov/books/NBK587351/.
7. Zhou P, Zhou J. The primary cilium as a therapeutic target in ocular diseases. Front Pharmacol 202;26;11:977.
8. Prosseda PP, Alvarado JA, Wang B, et al. Optogenetic stimulation of phosphoinositides reveals a critical role of primary cilia in eye pressure regulation. Sci Adv 2020;6.




