Rio de Janeiro, Brazil
Zdenek J. Gregor, FRCS, FRCOphth
London
Diabetic retinopathy is the leading cause of blindness among the working population in the developed world. The prevalence of diabetic retinopathy increases with duration of diabetes,1 and nearly 100 percent of patients with Type I diabetes and more than 60 percent of those with Type II have some signs of DR after 20 years. A number of approaches have proved to be useful in the treatment of DR, such as laser photocoagulation and tight systemic control of blood glucose, lipids, cholesterol and blood pressure.2,3 Unfortunately, in many patients the retinopathy progresses in spite of the best efforts on the part of the patient and of the ophthalmologist. Many such eyes may be helped by vitrectomy surgery, however.4 About 5 percent of patients with proliferative diabetic retinopathy, as well as carefully selected patients with diabetic maculopathy, require pars plana vitrectomy, despite ostensibly adequate laser treatment and good glycemic and hypertensive control.5
This article reviews the current indications for vitreous surgery in severe diabetic retinopathy and strategies and techniques employed to minimize surgical complications.
Pathogenesis of Diabetic Retinopathy
Diabetic retinopathy is a microvascular complication of diabetes and is characterised by the loss of pericyte function and by progressive capillary occlusions leading to retinal ischemia and breakdown of the blood-retinal barrier. This can result in edematous changes in non-proliferative diabetic retinopathy (NPDR) and in proliferation of new blood vessels and in the formation of contractile epiretinal fibrocellular membranes in PDR.6,7
The visual loss in PDR is caused by the combination of retinal ischemia, vitreous hemorrhage and/or traction retinal detachment (TRD). It usually begins as neovascular proliferation at the optic disc (NVD) and elsewhere (NVE). The growth of new vessels (NV) occurs, together with fibroblastic proliferation, at the vitreoretinal interface, using the posterior hyaloid as a scaffold.8 Subsequent contraction of the fibrocellular membranes causes progressive traction on NV, resulting in intragel and/or retrohyaloid hemorrhage. More severe and widespread traction leads to a TRD or a combined traction-rhegmatogenous retinal detachment (C-TRRD).9
Indications for Surgery in PDR
Vitrectomy for the complications of PDR was first described more than 25 years ago.10 PPV allows the removal of opacities from the media, such as non-clearing vitreous hemorrhage, and offers relief from vitreoretinal traction. In addition, peroperative retinal laser photocoagulation helps to stabilize the intraocular vasoproliferative process.9,11 Ultrasound examination in patients with dense opacities in the media is useful in making a preoperative diagnosis of retinal detachment and to differentiate between TRD and C-TRRD. Although visual outcomes of vitrectomy for PDR vary, most patients benefit from the procedure. 13
Vitreous Hemorrhage
Vitreous hemorrhage is a major complication of PDR, which can result in a severe reduction of visual acuity and may interfere with examination and treatment. In patients with Type II diabetes, a recent VH may be managed conservatively in the hope that spontaneous clearing occurs and adequate laser treatment can be applied. Persistent non-clearing VH (longer than three months) may require PPV and endolaser photocoagulation. In contrast, the Diabetic Retinopathy Vitrectomy Study (DRVS) showed a clear benefit from earlier surgery in patients with Type I diabetes, as these tend to develop more aggressive fibrovascular proliferation.14,15 The DRVS showed that 25 percent of patients vitrectomized early regained a good visual acuity of 20/40 or greater compared with 15 percent treated conventionally.15 There was, however, a higher treatment risk, but it should be kept in mind that surgical techniques have changed considerably since conclusion of the study (e.g., use of endolaser coagulation).
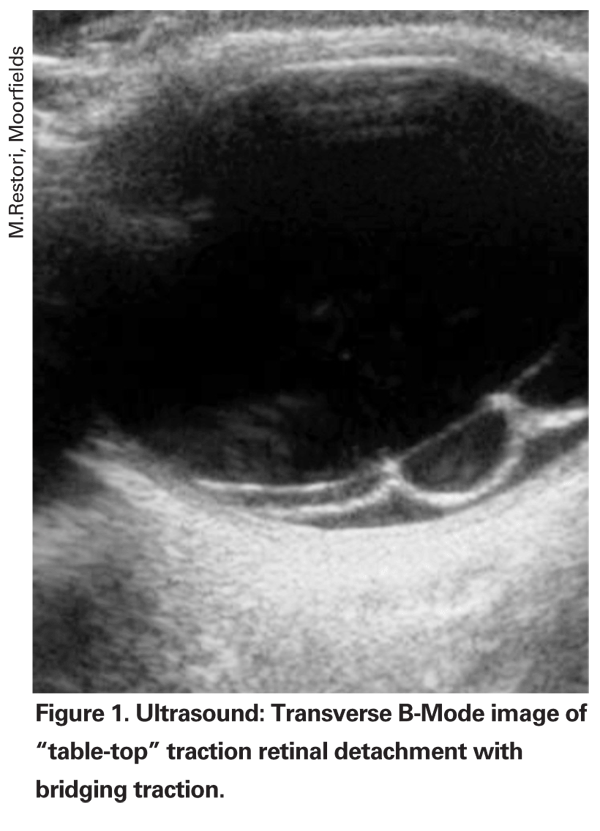
An earlier vitrectomy can also be considered in cases of retrohyaloid VH, as in this location the blood tends to absorb more slowly than when the blood has broken through the posterior hyaloid into the vitreous gel. The optimal time for surgery is also influenced by the condition of the fellow eye and any coexisting conditions, such as TRD involving the macula and/or neovascular glaucoma (See Figure 1). In the latter situation, waiting for clearance of the haemorrhage may cause irreversible damage.
Other factors that may dictate earlier surgery are the lack of adequate prior panretinal photocoagulation and, occasionally, patients needing faster visual recovery (e.g., work that requires good stereopsis).16
Retinal Detachment
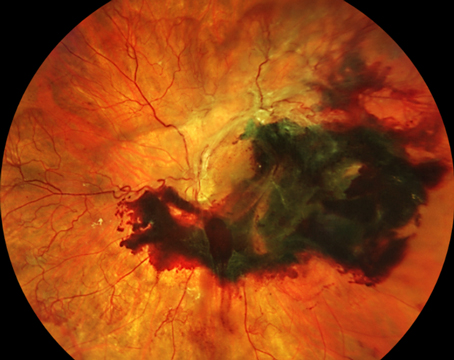
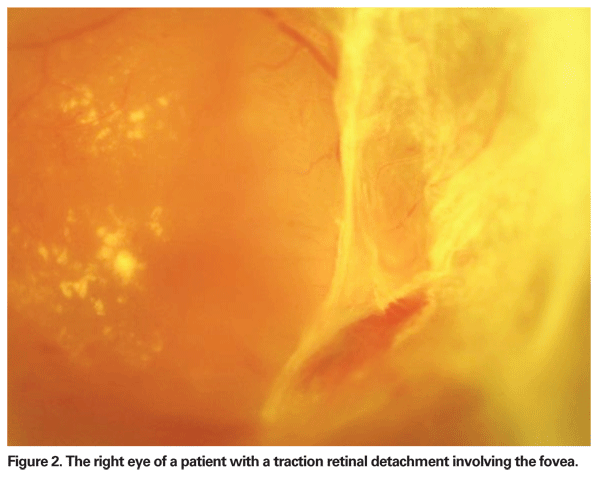
TRD involving the fovea causes severe visual loss and is now the commonest indication for surgery in patients with PDR (See Figure 2). A recent publication reported that in eyes with a TRD involving the macula, 57 percent achieved vision of 6/60 or better whereas in eyes without a macular detachment, 84 percent could see 6/60 or better.13
The urgency for surgery varies from patient to patient, but in most patients such operations are best planned for when appropriate facilities and operating theatre staff are readily available. Eyes with a recent history of foveal detachment have a better chance of visual recovery when compared to long-standing ones even if an equally good anatomical result is obtained.17
TRDs outside of the macular area can usually be safely monitored conservatively as they may remain stable and the risk of intra- or postoperative complications may be unacceptable.18 Another study showed that only 14 percent of eyes with extramacular tractional retinal detachment experienced a loss of vision within one year.19 However, PPV should be occasionally considered even before macular detachment occurs where adequate laser treatment cannot be applied because of VH or fibrovascular tissue covering the retina, or where progression of paramacular traction detachment has been documented.
C-TRRD in PDR is relatively uncommon and may occur either during the stage of active fibrovascular proliferation or as a late complication. In most cases, the retinal breaks are located posterior to the equator and are frequently associated with tightly adherent preretinal fibrovascular membranes. This type of detachment progresses rapidly and results in a bullous configuration of sub-retinal fluid extending to the ora serrata. Prompt PPV is usually necessary in order to relieve all the vitreoretinal traction, to apply laser treatment around retinal tears once the retina is flattened using intraocular tamponade, such as air or gas.20
Indications for Surgery in NPDR
Macular edema. Diabetic macular edema (DME) or retinal thickening is the leading cause of visual impairment in patients with diabetes mellitus. While the pathogenesis of DME is multifactorial, the vitreous may contribute to the development of DME in certain cases.21
Vascular leakage and ischemia are responsible for a greater part of the visual loss related to diabetic maculopathy. Vascular endothelial growth factor, considered the most important growth factor, causes the breakdown of the inner blood-retinal barrier, resulting in abnormal retinal vascular permeability and retinal edema.22 While a healthy retina also contains VEGF, diabetes-induced changes cause its upregulation.23 Indeed, VEGF levels have been shown to be elevated in eyes with DME.24
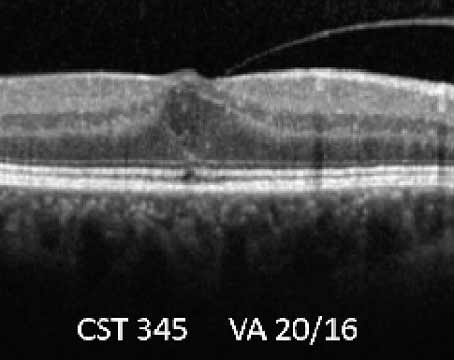
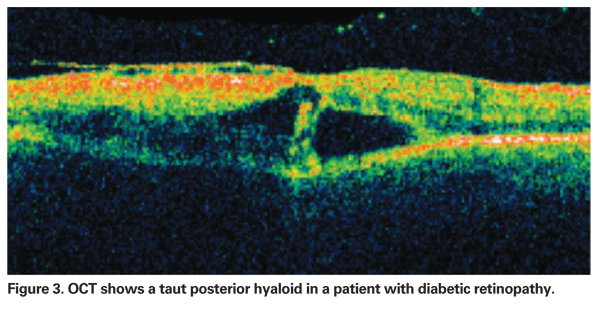
The role of the vitreous on the development of DME is unclear, but it is intriguing that natural history studies have shown that macular edema is more likely to resolve following spontaneous vitreomacular separation. Whereas PPV has been shown to result in anatomical and visual improvement in eyes with a taut posterior hyaloid membrane (TPHM) and demonstrable traction on the macula (See Figure 3),21,25 other studies suggested that PPV might also be useful in cases without any clinical evidence of traction. It may be that the removal of the vitreous cortex, with or without the peeling of the internal limiting membrane, releases sub-clinical vitreo-macular traction, improves retinal oxygenation and/or reduces the concentration in the posterior segment of growth factors (e.g., VEGF) and other cytokines released from the ischemic retina.26,27
In a randomized controlled trial of PPV with ILM peeling versus continuing focal laser photocoagulation, there was no difference in visual or anatomical results between the two treatment modalities.28 A randomised pilot study of patients with DME without macular traction also showed no visual benefit or reduction in macular thickness.29
Surgical Techniques
When VH is present, a standard PPV is performed first and the posterior hyaloid face (PHF) is identified; if there is a significant amount of blood behind the PHF, an opening in the PHF is created in order to aspirate the retrohyaloid blood and to gain adequate view of the retina.30 It is imperative to release any traction on the retina by existing membranes. Such membranes are almost always vascularized, and thus they cannot be simply peeled from the surface of the retina, as this would result in severe hemorrhage and/or tearing of the retina.30,31 Segmentation, delamination, en-bloc delamination and bimanual dissection represent the main surgical techniques employed in diabetic vitrectomy.
Segmentation. Segmentation involves the vertical cutting of epiretinal membranes into small segments, and this can be accomplished either with vertically cutting scissors or a mechanized vitreous cutter. Segmentation is used to release circumferential traction when other methods, such as delamination, are made difficult by very mobile retina in the presence of a retinal break. When segmenting membranes, it is not necessary to remove the membranes completely, leaving small, circumscribed remnants centered on the neovascular pegs. The disadvantage of the segmentation technique is that the residual islands of fibrovascular tissue may encourage reproliferation and recurrent bleeding.30,31
Delamination. The risk of postoperative bleeding may be reduced by a complete removal of fibrovascular tissue from the retina using horizontally cutting scissors to sever the individual neovascular pegs from the retinal surface. The aim is to cut rather than avulse the neovascular pegs, as this would lead to perioperative hemorrhage from the sidewall of a retinal vessel, which may prove to be difficult to control. Finding the correct plane between the posterior hyaloid and the retina near the vascular epicenter is crucial, in order to avoid iatrogenic tears.30-32
En-bloc delamination is preferable to segmentation because it enables us to completely remove all the fibrovascular tissue from the retinal surface.30 In this technique, the posterior hyaloid is kept partially intact in order to use the continuing antero-posterior traction to elevate the epiretinal membranes during dissection. A small window in the partially detached posterior hyaloid is made so that a horizontally cutting scissors can be introduced into the retrohyaloid space. Gentle traction immobilizes the fibrovascular membrane and the underlying elevated retina exposing areas of adhesion between the membrane and the retina facilitating its excision. The light pipe or illuminated forceps can be used to turn the membrane over and facilitate the dissection. When the membranes have been completely separated from the retina the remaining posterior hyaloid complex can be removed with the vitreous cutter.
Techniques for C-TRRD may require the combination of delamination and segmentation techniques, because of the difficulties in completely separating the fibrovascular membranes from a mobile detached retina. Bimanual surgical technique can be employed in such complex cases using a separate light source, which allows the surgeon to use two instruments for dissection.30
Laser treatment. Endolaser photocoagulation is always applied even in eyes with pre-existing PRP, in order to decrease the neovascular drive and thus minimize recurrent or delayed hemorrhage. A 360-degree search of the entire retina is made before the end of the surgery, in order to identify any pre-existing or iatrogenic breaks (e.g. posterior tears or entry site breaks). If found they must be treated with either laser photocoagulation or cryotherapy in conjunction with a tamponading agent (e.g. air, expanding gas or silicone oil). In a prospective study of 174 consecutive vitrectomies, 39 percent of eyes were found to have a retinal break. In 27 percent the breaks were posterior and occurred during membrane dissection, whereas 17 percent of such eyes had entry site breaks. Altogether 49 percent of eyes required internal tamponade, with air used in 8 percent of eyes, SF6 gas in 24 percent, C3F8 gas in 10 percent and silicone oil in 7 percent.13
Vitrectomy for DME. Standard PPV for DME may be performed with or without the peeling of the ILM. Dyes, such as indocyanine green and Membrane Blue, can be used during PPV to stain the ILM and improve its visualization.
A comparative study of vitrectomy for diffuse clinically significant macular edema demonstrated structural improvement—in foveal thickness and significant improvement in the macular volume—but limited visual improvement after ILM peeling 12 months postop.33
Antiangiogenic Therapy
Several clinical trials are currently evaluating the role of anti-VEGF agents for the treatment of ocular diseases associated with intraocular neovascularisation and/or retinal vascular leakage. The use of anti-VEGF drugs has been recently added to the therapeutic armamentarium for neovascular complications of PDR.34,35 Several case series have suggested the use of intravitreal bevacizumab (Avastin) as a helpful preoperative adjunct in patients with TRD and actively proliferating new vessels as it causes rapid involution of NV both in the anterior and posterior segments.36,37
Surgical Complications
Immediate postoperative hemorrhage is quite common and usually resolves spontaneously over a few weeks. Delayed postoperative hemorrhage occurs in some 10 percent of diabetic eyes, and this can be related to neovascular proliferation in the vitreous base, often at the vitrectomy entry sites. A recent publication showed that the most common postoperative complication was vitreous cavity hemorrhage, which occurred in 22 percent of eyes.13 Anterior cryotherapy or confluent laser posterior to the ora serrata may reduce the risk of fibrovascular ingrowth and recurrent hemorrhage.38
Periodic ultrasound examinations in eyes with no fundal view should be performed to exclude retinal detachment. Rhegmatogenous retinal detachment after vitrectomy is usually due to unrecognized retinal breaks and requires urgent surgery to prevent otherwise rapidly developing proliferative vitreoretinopathy and/or rubeosis iridis.14,39
In another study a 4.7 percent incidence of retinal detachment was found.40 The risk of RRD following diabetic vitrectomy appears to be declining, probably due to the use of wide-angle viewing systems, which allow for more accurate examination of the peripheral retina.40
In the anterior segment, corneal epithelial defects have also become less common following the introduction of non-contact wide angle viewing systems. Although a nuclear cataract commonly develops after vitrectomy, this is less common in patients with diabetes. One publication reported an incidence of cataract extraction of 15 percent after diabetic vitrectomy compared with 66 percent and 53 percent after vitrectomy for macula hole or epiretinal membrane, respectively.42 Combined PPV with phacoemulsification and lens implantation ("phacovitrectomy") is gaining in popularity. However, it is not recommended for patients with severe retinal ischemia, rubeosis iridis and patients with severe forms of TRD.43
Mr. Gregor is a consultant ophthalmic surgeon at
1. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XII: The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801-1815.
2. DCCT Research Group. The effect of intensive treatment of diabetes in the development and progression of long-term complication in insulin-dependent diabetes. N Engl J Med 1993;329:977-986.
3.
4. Helbig H, Sutter FXP. Surgical treatment of diabetic retinopathy. Graefe's Arch Clin Exp Ophthalmol 2004;242:704-709.
5. Flynn HW Jr., Chew EY, Simons BD, Barton FB, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1992;99:1351-1357.
6.
7. Klein R, Klein B, Moss S, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, IV: Diabetic macular edema. Ophthalmology 1984;91:1464-1474.
8. Gotzaridis EV, Lit ES, D'Amico DJ. Progress in vitreoretinal surgery for proliferative diabetic retinopathy. Semin Ophthalmol 2001;16 (1):31-40.
9. Mason JO,
10. Smiddy WE, Flynn HW Jr. Vitrectomy in the management of diabetic retinopathy. Surv Ophthalmol 1999;43: 491-507.
11. Diabetic Retinopathy Study Research Group. Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Study Report #1. Ophthalmology 1985;92:492-502.
12. Thompson MJ, Ip MS. Diabetic macular edema: A review of past, present, and future therapies. Int Ophthalmol Clin 2004;44:51-67.
13. Yorston D, Wickham L, Benson S, Bunce C, et al. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol 2008;92: 365-368.
14. Diabetic Retinopathy Vitrectomy Study Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy Study Report 5. Arch Ophthalmol 1990;108:958-964.
15. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. The Diabetic Retinopathy Vitrectomy Study Research Group. Arch Ophthalmol 1985;103:1644-1652.
16. Michels RG. Proliferative diabetic retinopathy. Pathophysiology of extraretinal complications and principles of vitreous surgery. Retina 1981;1:1-17.
17. Cohen HB, McMeel JW, Franks EP. Diabetic traction detachment. Arch Ophthalmol 1979;97:1268-1537.
18. D'Amico DJ. Diabetic traction retinal detachments threatening the fovea and pan retinal argon laser photocoagulation. Semin Ophthalmol 1991;6(1):11-18.
19. Charles S, Flinn CE. The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol 1981;99:66-68.
20. Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous retinal detachment. Arch Ophthalmol 1987;105:503-507.
21. Hikichi T, Fujio N, Akiba J, Azuma Y, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology 1997;104: 473-478.
22. Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001;42:2408-2413.
23. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331:1480-1487.
24. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445-450.
25. Lewis H, Abrams G, Blumenkranz M, Campo R. Vitrectomy for diabetic macular edema associated with posterior hyaloid traction. Ophthalmology 1992;99: 753-759.
26. Patel JI, Hykin PG, Schadt M, Luong V, et al. Pars plana vitrectomy for diabetic macular oedema: OCT and functional correlations. Eye 2006;20:674-680.
27. Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy for diffuse nontractional diabetic macular edema. Retina 2008;28:420-426.
28. Kumar A, Sinha S, Azad R, Sharma YR, Vohra R. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefe's Arch Clin Exp Ophthalmol 2007;245:360-368.
29. Patel JI, Hykin PG, Schadt M, Luong V, et al. Diabetic macular oedema: Pilot randomised trial of pars plana vitrectomy vs. macular argon photocoagulation. Eye 2006; 20:873-881.
30. Aylward GW, Sullivan P, Vote B. The Video Atlas of Eye Surgery. Vitreoretinal-Basic Techniques. Eye Movies Ltd., 2005.
31. Kanski JJ, Gregor ZJ. Retinal Detachment: A Colour Manual of Diagnosis and Treatment. Butterworth-Heinemann, 1994, p. 161.
32. Schwatz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology 1996;103:323-328.
33. Patel JI, Hykin PG, Schadt M, Luong VY, et al. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina 2006;26:5-13.
34. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005; 36(4):331-5.
35. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous haemorrhage. Retina 2006;26:275-278.
36. Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefe's Arch Clin Exp Ophthalmol 2008;246:837-842.
37. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006;26:699-700.
38. Mason JO III,
39. Charles S. Vitreous Microsurgery. Baltimore, Williams and Wilkins, 1981, p. 115.
40. Schrey S, Krepler K, Wedrich A. Incidence of rhegmatogenous retinal detachment after vitrectomy in eyes of diabetic patients. Retina 2006;26:149-152.
41. Virata SR, Kylstra JA. Postoperative complications following vitrectomy for proliferative diabetic retinopathy with sew-on and non-contact wide-angle viewing lenses. Ophthalmic Surg Lasers 2001;32:193-197.
42. Smiddy WE, Feuer W. Incidence of cataract extraction after diabetic vitrectomy. Retina 2004;24:574-581.
43. Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefe's Arch Clin Exp Ophthalmol 2006;244:808-815.