The 2006 Physician Fee Schedule, published by the Centers for Medicare & Medicaid Services last month, contains an expected 4.4-percent across the board decrease in physician fees. The agency continues to use a Sustainable Growth Rate (SGR) that critics call flawed in determining the conversion factor—reducing it from $37.8975 in 2005 to $36.1770 in 2006. The repeal of the SGR is one of the Academy of Ophthalmology's highest policy priorities.
CMS decided not to move forward with changes to the way it calculates practice expense (PE) as set forth in the proposed 2006 Physician Fee Schedule rule. The PE, costs incurred in the operation of a physician's practice, represent 45 percent of the physician's fee. The changes proposed by CMS involved the acceptance of new data from six major specialties and would have cut ophthalmology's payments by another 4.4 percent, phased in over four years, starting with a 1.1 percent cut Jan. 1, 2006.
In the final 2006 Physician Fee Schedule, CMS announced that it would not go forward with the PE proposal, and it will continue to use 2005 PE rates to value all services in 2006. CMS is expected to develop a multi-specialty indirect PE survey as recommended by the AAO and a coalition of medical interest groups.
"The PE refinement that targeted ophthalmology for big cuts—a total of $474 million over four years—was the key component in the rule that we could affect," said William Rich III, MD, the AAO's medical director of health policy. "I am pleased to see that this effort, led by the academy, was successful."
Other positive changes are set out in the fee schedule. One is the expansion of Medicare coverage of glaucoma screening to include Hispanic Americans age 65 and older. CMS also responded to AAO requests to add Visudyne (verteporfin) and Macugen (pegaptanib) to the list of Competitive Acquisition Program (CAP) approved drugs. The AAO worked to ensure that ophthalmologists would have the option of participating in and procuring these costly drugs through the CAP program.
WaveLight Presents Custom Results
Investigators in the clinical trial for the Allegretto Wave custom-guided system recently submitted their data to the U.S. Food and Drug Administration, and discussed the three and six-month results at October's American Academy of Ophthalmology meeting. They also compared the data to that from their wavefront-optimized system (which is currently approved). Wavefront optimized procedures don't use a patient's particular wavefront profile to plan an ablation, but instead use beam profiles that were created with optimal wavefronts in mind and strive to maintain the cornea's natural aspherical shape postop to avoid spherical aberration problems. The Allegretto Wave wavefront-guided procedures use each patient's wavefront to plan his or her procedure.
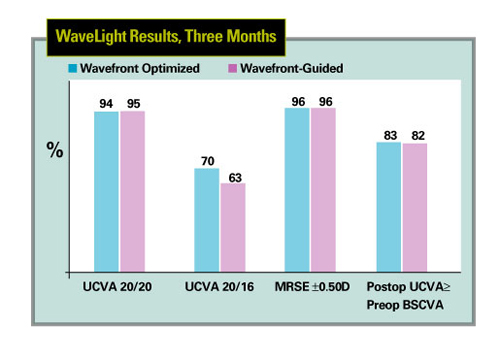 |
| For myopes up to -7 D with up to 3 D of cylinder, wavefront-guided results with the Allegretto laser were very similar to wavefront-optimized outcomes. |
In a three-month analysis of the results performed by WaveLight investigator Karl Stonecipher, MD, wavefront-optimized and wavefront-guided ablations were very similar.
In the WaveLight FDA trial, at three months, 348 eyes were available for follow-up. Preop, the eyes had up to 7 D of myopia with up to 3 D of cylinder. Half were randomized to receive wavefront-optimized treatment, and half to receive the new wavefront-guided procedure. Postop, 94 percent of 174 wavefront-optimized eyes saw 20/20 or better, compared to 95 percent of the 174 wavefront-guided cases. The same percentage in each group, 96 percent, were within ±0.5 D of the target refraction postop. Eighty-three percent of the optimized group and 82 percent of the custom group had a postop uncorrected acuity that was actually better than or equal to their preop best-corrected acuity.
The investigators found it interesting that not until the root mean square value of patients' aberrations reached 0.3 µm at a 6-mm pupil did the customized treatment start to trend toward better outcomes. The difference didn't become really meaningful until the RMS was 0.4 µm or higher.
"The only way we could find that custom ablation did better than optimized to a statistically significant degree was if you had higher-aberrations of 0.3 µm or more," says Dr. Stonecipher. "And that was pretty weak. At 0.4 µm or more, we saw a statistically significant difference. Now, having said that, 81 percent of the patient population had higher-order aberrations of less than 0.3 µm, and 96 percent of the patients had aberrations less than 0.4 µm. So, despite there being some mild favor for wavefront-guided treatments of the WaveLight in these patients, it's probably only in the neighborhood of 5 to 10 percent of the population that needs wavefront-guided treatment." Wavefront-guided treatments mainly showed a benefit in low-contrast visual acuity.
"We found an improvement in low-contrast acuity in the wavefront-guided group, but only at the 0.4-µm RMS level or higher," says Dr. Stonecipher. "When evaluating ETDRS visual acuity or contrast sensitivity there were no significant differences between the groups. Also, subjective patient questionnaires showed no differences."
Another result that intrigued Guy Kezirian, MD, whose firm SurgiVision Consultants oversaw the trial, was that in both cohorts, if a patient had more than 0.3 µm of higher-order aberrations, the average higher-order RMS decreased. They found that the reduction was more in the wavefront-guided group, but was still statistically significant for both groups.
The application for approval is with the FDA, and the company hopes to have an FDA decision by mid-2006.
"[The results] don't mean that wavefront-guided treatments are a waste of time," notes Dr. Kezirian. "And, it doesn't mean that aberrations don't cause symptoms. It just means that, with this platform, wavefront-guided and wavefront-optimized treatments result in vision that is below the threshold for causing symptoms at night."
Glaucoma Risk Calculator Debuts
A new glaucoma risk calculator designed to help physicians determine whether to initiate therapy for patients with ocular hypertension debuted at October's American Academy of Ophthalmology meeting. Robert N. Weinreb, MD, and Felipe A. Medeiros, MD, developed the risk calculator, which estimates the risk of developing glaucoma for patients with ocular hypertension.
"The risk calculator helps to identify patients with ocular hypertension who are at high-risk of developing glaucoma and may benefit from treatment," said Dr. Weinreb, director of the Hamilton Glaucoma Center and professor of ophthalmology at the University of California, San Diego. "It will allow doctors to assess patient risk levels and, if needed, recommend treatment options that can help avert possible progression to glaucoma."
The pair subsequently validated the model in patients taking part in the Diagnostic Innovations in Glaucoma Study (DIGS), an ongoing prospective study at the Hamilton Center.
Patients with ocular hypertension may have multiple risk factors for progression to glaucoma. A collective assessment of these risk factors helps clinicians identify patients with elevated eye pressure who are more likely to progress to glaucoma and may benefit from early treatment. Similar predictive models have a history in other therapeutic areas, such as the cardiac risk assessment that was developed in the Framingham Study in the 1950s.
Drs. Weinreb and Medeiros took key findings from OHTS, which identified important patient risk factors predictive of disease progression from ocular hypertension to glaucoma. These included older age, elevated IOP, thinner central corneal thickness, increased vertical cup/disc ratio, and greater pattern standard deviation. The risk calculator, which resembles a slide rule, allows physicians to assess the six risk factors and position their findings at various points on the tool. When taken in combination, these factors help determine the risk of conversion from ocular hypertension to glaucoma within the next five years.
Under the risk calculator guidelines, patients with a determined progression risk of less than 5 percent should monitor their condition with their doctor. Consideration and discussion of benefits and risks of treatment with a physician are recommended for individuals determined to have a 5 to 15 percent risk of progressing to glaucoma, while treatment is recommended for those with higher levels of risk.
"The risk calculator should be used as an adjunct to, and not as a substitute for, clinical experience and judgment, and each physician is likely to have his or her own threshold for treatment," said Dr. Medeiros, assistant professor of ophthalmology at UC, San Diego. "Other factors such as the patient's overall health status, life expectancy and commitment to treatment should also be weighed against potential adverse events and cost of treatment."
Bacterium Found in AMD Eyes
Researchers at the Massachusetts Eye and Ear Infirmary have found that Chlamydia pneumoniae, a bacterium linked to heart disease and capable of causing chronic inflammation, was present in the diseased eye tissue of five out of nine people with neovascular age-related macular degeneration. It was not found, however, in the eyes of more than 20 individuals without AMD, providing more evidence that this disease may be caused by inflammation. The study is described in the November issue of Graefe's Archive for Clinical and Experimental Ophthalmology.
Researchers at MEEI and Harvard Medical School examined nine wet AMD membranes for the presence of C. pneumoniae and also determined whether this pathogen can change the function of eye cells in ways that can cause wet AMD. They found C. pneumoniae in the eyes of five of nine patients with wet AMD. They also tested tissue from more than 20 people who did not have AMD and did not find C. pneumoniae in any of these normal eye tissues.
"The paper showed that C. pneumoniae is capable of modifying the function of important cell types involved in regulating normal eye function," said lead author Murat Kalayoglu, MD, PhD. The researchers reported that C. pneumoniae infection led to increased production of vascular endothelial growth factor, the key protein involved in wet AMD. That C. pneumoniae infection of human eye cell types increases VEGF production is therefore significant and could explain in part why VEGF levels are increased in many people with wet AMD.



