It’s no secret that patients dislike the protocol they have to undergo following retinal detachment surgery, with its requirement for very uncomfortable positioning—not to mention the postop visual side effects and travel restrictions. This reality has inspired a number of researchers to try to develop alternatives that might eliminate these downsides.
Among those working to solve this problem are Tomasz (Tommy) Stryjewski, MD, and Tony Stefater, MD, PhD, two young retinal surgeons in Boston. Here, they explain how this problem caught their attention, and where their work has led so far.
Getting on the Trail
“After retinal surgery, if there are any breaks or holes in the retina, you have to fill the eye with something that’s going to keep the hole sealed while the laser sets,” Dr. Stryjewski explains. “For more than 40 years ophthalmologists have been using special gases and hydrophobic oil to create a seal. Unfortunately, if you have any kind of gas or oil inside your eye, your vision is quite poor—unlike after cataract surgery, when patients can see quite well from day one. That’s a very big problem, especially if it’s your only eye. In addition, you have to position your body in ways that can be very uncomfortable, and you have limitations such as not being able to travel via airplane because of the air pressure changes flying entails.
“We became interested in this problem when we were both residents at Mass Eye and Ear,” he explains. “We marveled that patients didn’t complain about their eye after retinal detachment surgery; instead, they complained about their neck and back and how uncomfortable they were with the positioning they underwent. We wondered if we could come up with a tamponade material that could provide a seal against the retina and then degrade, without all of the side effects and limitations. Patients could have a much better experience after retinal surgery.”
 |
| This new retinal tamponade gel will remain clear inside the eye until it breaks down and leaves through the eye’s normal outflow system after about a month. |
Dr. Stryjewski says they began working on the problem five or six years ago. “We envisioned ourselves eventually becoming academic physicians working on NIH grants,” he says. “We were passionate about doing research and contributing to the fund of knowledge. But because we were in Boston working at this big life-science biotech hub, we got interested in the idea of starting a company to develop this. As a result, we decided to found Pykus Therapeutics, with the goal of trying to develop this type of technology for patients.”
Dr. Stryjewski explains that their initial funding came from winning a business competition put on by Harvard. “Eventually, we were able to raise enough capital to hire a team of chemists, product and development specialists, and regulatory experts. Then, we established a laboratory in the Boston area, where we’ve conducted our early research using an animal model.”
Dr. Stefater acknowledges that other researchers are striving to reach the same goal. “We’re not the first people to think of this,” he notes. “There’s a lot of published literature about work done in animals and other preclinical models using hydrogels and other substances to treat retinal detachments. But for a variety of reasons, none of them have advanced to the clinic. It turns out to be a challenging problem.” They note that seemingly promising alternatives tried by others that have not made it to the clinic so far have included heavy silicone oils, magnetic oils, combination gas-oils, retinal sealants, intraocular capsules and hydrogels.
Addressing the Challenge
“In the past few years we’ve had first-hand experience with all of the issues that surround developing a novel tamponade agent for the retina,” Dr. Stefater explains. “There are three problems that are especially thorny: First, many molecules and compounds are inflammatory in the eye. Second, the product should ideally degrade inside the eye so it doesn’t have to be removed. That means that the substance has to clear through the eye’s normal clearance system without causing elevated pressure.
“The third major problem relates to getting the product into the eye,” he continues. “Retinal surgery has gotten safer and safer because it’s now done through very tiny incisions, but that’s a challenge if you want to inject a hydrogel into the eye. If you push gelatin through a syringe, for example, it’s not going to be solid when it comes out of the needle. So how do you get a substance that’s rigid enough to support the retina into the eye through a tiny incision? Actually, several groups, including ours, have begun to make real progress solving that last problem. We’ve been developing polymers that are liquid outside the eye but form a hydrogel inside the eye.”
Dr. Stryjewski says they identified a class of molecules that performs the way they needed it to. “However, there were still plenty of challenges,” he notes. “For example, how do you store a material with such unusual properties? Will it remain stable? The materials we looked at worked well, but in some cases they couldn’t be stored on the shelf. Or, there would be issues of solubility; you might need to warm it up in a glass beaker and stir it to get it to dissolve before injection into the eye. That’s not practical for a practicing clinician. Our goal has been to create a product with clinical use requirements that would be familiar to surgeons, not exotic. So a lot of the challenge has been marrying the ideal chemical properties with a product that’s manageable in the clinic.
 |
| Click image to enlarge. |
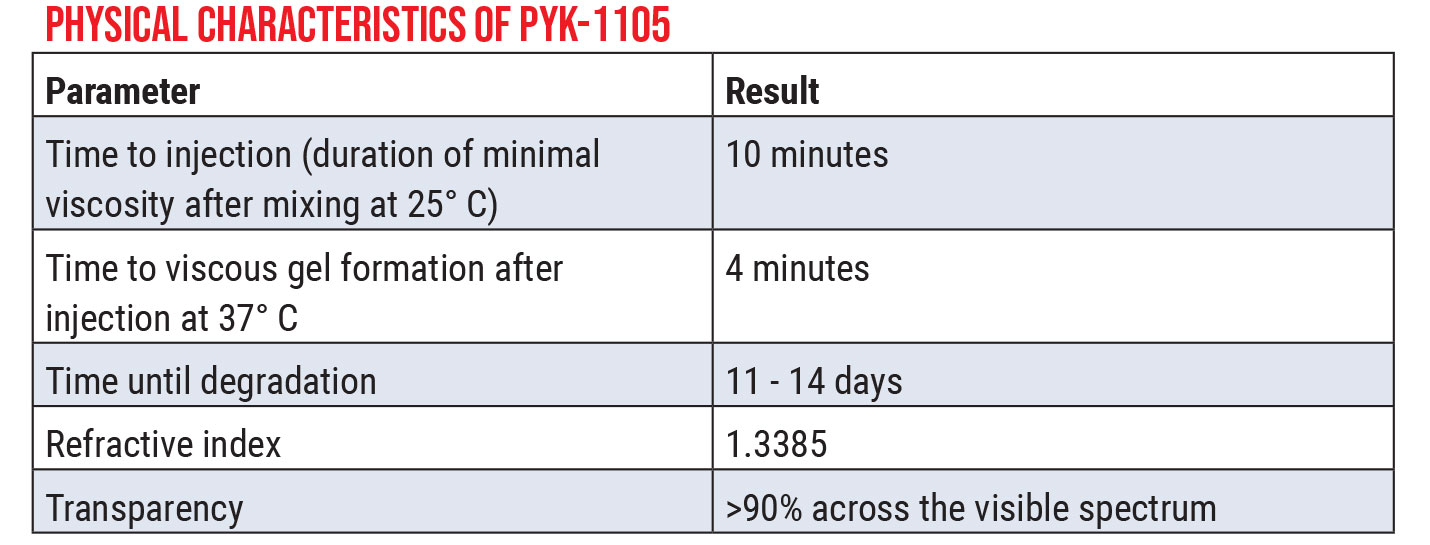
“That’s where very experienced formulation chemists are really valuable,” he says. “They’ve helped us achieve the goal of creating a product that’s shelf-stable and can be easily handled in the OR in a way that’s familiar to surgeons. This product (PYK-1105) comes in two parts that are mixed; it forms a thick, viscous liquid that rapidly turns into a soft gel when it reaches body temperature.
“We consider ourselves to be retina surgeons first and foremost,” he concludes. “We’ve come to appreciate the tremendous amount of work it takes to create a product that’s clinically feasible. There’s a tremendous gulf between an interesting science project and something that’s clinically practical.”
Dr. Stryjewski explains how the product would be used in the clinic. “Tamponades function by keeping the hole in the retina dry, blocking water from accessing the subretinal space while the laser scar sets,” he says. “With our product, the retina would be repaired in the usual manner. A fluid-air exchange would be performed; the tear would be lasered. Once the retina is flat under air and you’ve applied your laserplexy, the gel would be added to the eye in much the same way surgeons currently inject an oil tamponade, and the gel would set on top of the break. Once the gel has been applied, the case is over.
“The gel would remain in the eye for about a month,” he continues. “In contrast to oil and gas, the patient will be able to see through this substance. After about a month, the gel will break down and leave the eye. In the meantime, the gel would keep the repair dry and the laser treatment would be able to set.” Dr. Stefater adds that they anticipate that the product will be covered by insurance, making it available to everyone.
The Future Looks Bright
Dr. Stefater points out that one reason this problem has remained unaddressed for so long is that using gas for retinal tamponade works well—at least from the surgeon’s perspective. “The burden isn’t really on the surgeon, however,” he notes. “It’s on the patient who has to position face-down for a week. When you talk to patients, they’ll often tell you it was the worst week of their lives. It’s hard to imagine how unpleasant it is if you haven’t gone through it.
 |
| The new substance is liquid outside the eye, but forms a hydrogel inside the eye that can protect the retina while it heals. |
“The next phase of retinal surgery evolution will hopefully eliminate that patient burden,” he continues. “Patients will be able to see after surgery; they’ll be able to fly; and they won’t have to position face-down. It would definitely make it a much better experience. But of course, there are many challenges to making this a reality; that’s why it’s taken us several years to get to this point. And it will be a little while longer before we have a product that gives patients a perfect postop experience.”
Their preclinical studies demonstrated that the current formulation is well-tolerated in a rabbit and mini-pig vitrectomy model. “After presenting a summary of our pre-clinical work at the 2019 Vail vitrectomy meeting and the 2020 Retina Society meeting and publishing in the Journal of Vitreoretinal Diseases, we’ve been able to launch our first human study this year,” Dr. Stryjewski notes. “We’re recruiting 10 retinal detachment patients with low visual potential; the study is ongoing. As you can imagine, we’ve spent much of this year navigating the challenges involved in conducting a study during a pandemic. It’s too early to offer any details about our early findings, but there have been many encouraging signs that we’re on the right track. We hope to complete enrollment in the pilot clinical study this year.”
“Everyone working on this problem wants to help patients recover more easily from retinal detachment surgery,” Dr. Stryjewski concludes. “My hope is that, whether it’s our product or someone else’s, a generation from now patients undergoing retinal surgery will have a much more comfortable postoperative experience."
Dr. Colvard is a surgeon at the Colvard-Kandavel Eye Center in Los Angeles and a clinical professor of ophthalmology at the Keck School of Medicine of the University of Southern California. Dr. Charles is the founder of the Charles Retina Institute in Germantown, Tennessee.
Dr. Stryjewski is in private practice at Tallman Eye Associates (Lawrence, Mass.); Dr. Stefater is in private practice at Ophthalmic Consultants of Boston. Drs. Stryjewski and Stefater are cofounders and equity co-owners of Pykus Therapeutics, which is developing this technology for clinical use.