Diagnosing and treating pediatric glaucoma presents many challenges that differ from those facing ophthalmologists treating adult glaucoma. For example, it’s notoriously easy for an adult patient to have glaucoma without realizing it; the glaucoma is usually symptomless except for vision loss after the glaucoma has gotten pretty advanced. In contrast, very young children with glaucoma tend to display symptoms.
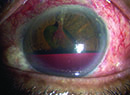
Because the eye is growing larger during infancy, it’s somewhat stretchable, and glaucomatous pressure can cause it to enlarge. In addition, the cornea can swell and become cloudy. (Severe cloudiness may indicate a condition such as Peters’ Anomaly rather than glaucoma, but like other congenital eye conditions, Peters’ Anomaly can eventually lead to glaucoma.) The eye may tear, and the child may become sensitive to light. (On the positive side, the fact that early childhood glaucoma often produces symptoms can cause parents to notice that something is wrong.)
Several issues arise when we’re faced with an infant or child with possible glaucoma. Those include getting a measurement of the IOP (not always easy to do with a young child); diagnosing the problem; deciding whether to address the glaucoma with topical drops or surgery; and deciding whether—and how often—anesthesia can safely be used. Here, I’d like to share some of my experience dealing with these issues.
Childhood Glaucomas
Primary congenital glaucoma is the most common type of childhood glaucoma. PCG is caused by a con-genital anomaly, and in the United States it appears in one out of every 10,000 to 50,000 children and is usually sporadic. It’s more common in some other countries where con-sanguineous marriage is accepted, because it’s tied to an autosomal recessive gene. In that setting, PCG can appear in as many as one out of every 2,500 children.
The other type of childhood glaucoma we most often treat—though less often than PCG—is glaucoma following congenital cataract surgery. This is actually less common in developing countries than it is here, simply because it’s tied to the timing of the congenital cataract surgery. The later the cataract surgery is done, the less likely the patient is to have a good visual outcome—but the odds of post-surgical glaucoma decrease as well. (I’m currently working on the 10-year Infant Aphakia Treatment Study; the five-year data confirm that perform-ing unilateral cataract removal at a younger age increases the risk of glaucoma-related adverse events.1) In developing countries, diagnosis and referral of congenital cataract is often delayed, lowering the likelihood of postsurgical glaucoma. In the United States and Europe, where childhood cataracts tend to be caught and treated earlier, glaucoma following congenital cataract surgery is pretty common.
Beyond these two types of pediatric glaucoma, there are a variety of others related to other defects of the eye. Basically, if something affects the anterior segment of the eye during development, there’s usually an association with glaucoma, and some childhood glaucomas are associated with systemic diseases or syndromes. These are less common than the first two types I described, but they do occur—and they’re often more challenging to treat.
Pediatric Glaucoma Reclassified
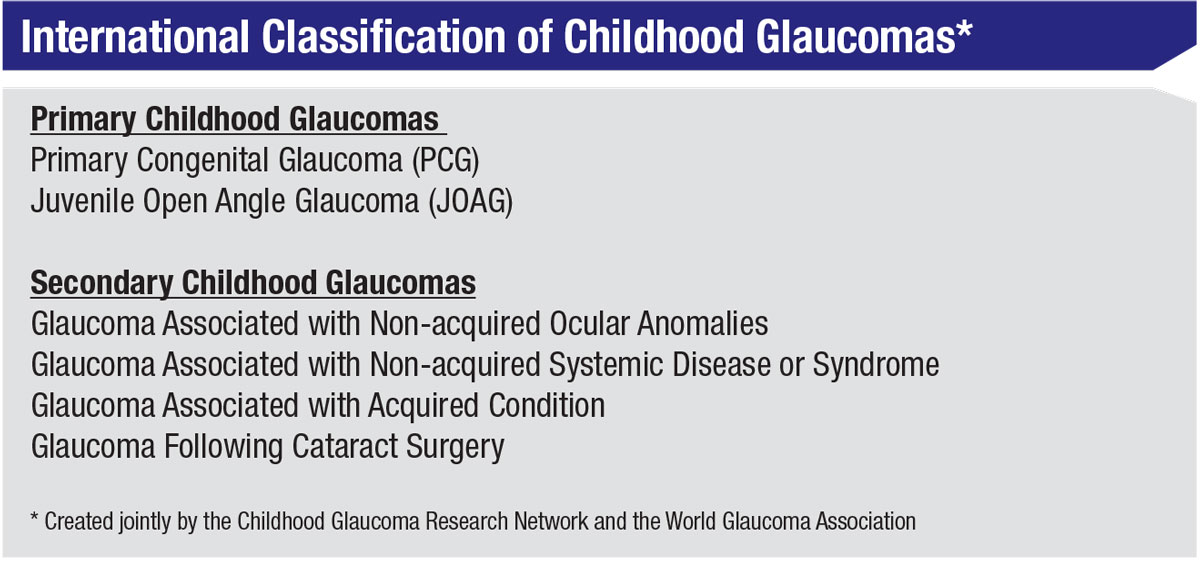 |
It’s worth noting that the classification of childhood glaucoma has changed over the past decade. The previous classification system was very involved and detailed; it listed any condition that could be associated with childhood-onset glaucoma. The new classification system (right), created jointly by the Childhood Glaucoma Research Network and the World Glaucoma Association, is an attempt to put those conditions into larger groups, based on related mechanisms. This is especially helpful when studying pediatric glaucoma, because many of the glaucomas are so rare that you can’t easily compare one to another unless you group them by related mechanisms.
The new system divides pediatric glaucoma into two types: primary and secondary. Primary childhood glaucomas include primary congenital glaucoma and juvenile open-angle glaucoma. Juvenile open-angle glaucoma is an autosomal dominant form caused by a mutation in the myocilin gene; it tends to run in families. The eye looks normal, but the fluid drainage doesn’t function normally. It’s very aggressive, occurring in child-hood or early adulthood, and without surgical intervention it frequently results in blindness. In childhood it’s less common than primary congenital glaucoma, but as noted, it often presents in early adulthood.
Secondary childhood glaucomas include:
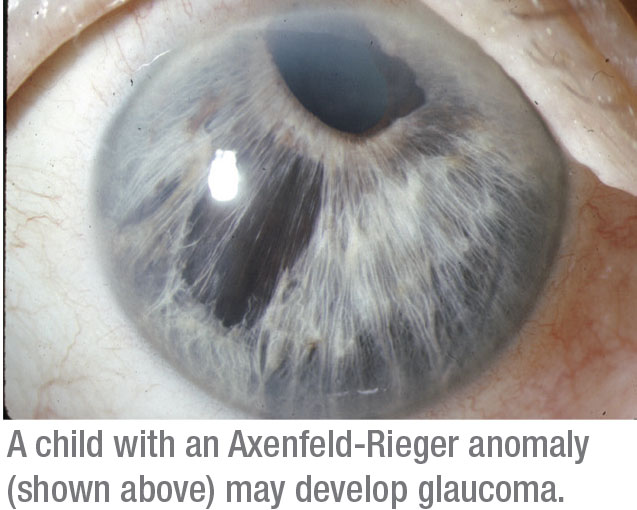
• Glaucomas associated with non-acquired ocular anomalies. This category refers to a number of different front-of-the-eye disorders present at birth, such as aniridia or Axenfeld-Rieger anomaly. Roughly 50 percent of patients with an abnormality in the front part of the eye end up with glaucoma.
• Glaucomas associated with a non-acquired systemic disease or syndrome. Childhood glaucoma can be associated with systemic diseases and syndromes present since birth, such as congenital rubella. In a developing country, where immunization is less widespread, congenital rubella is actually a fairly common cause of secondary glaucoma.
• Glaucomas associated with an acquired condition. This refers to something that happens after birth, such as trauma to the eye, uveitis or retinopathy of prematurity. (In ROP, although the baby is born prematurely, the disease develops afterwards, and it can cause a secondary angle-closure form of glaucoma.) Less-common conditions such as tumors can also be associated with childhood glaucoma.
• Glaucoma that occurs following congenital cataract surgery. Glaucoma following cataract surgery is technically an acquired condition, but it’s so important that we made it a separate category. In these patients, the cataract is there at birth, but the surgery is done after birth. We know it’s the surgery that induces the glaucoma, because if you don’t operate on the cataract, glaucoma usually doesn’t occur.
Differential Diagnosis
Diagnosing childhood glaucoma can be tricky, in part because other conditions can mimic it by making the cornea cloudy or causing symptoms such as tearing. Problems that can cause corneal clouding include birth trauma, Peters’ anomaly, and sclerocornea and lysomsomal storage diseases. Birth trauma can be caused by forceps delivery, and this can also mimic glaucoma. If the forceps happens to clamp across the baby’s head and eye, it can damage the cornea and cause swelling. (The swelling eventually resolves, but it can lead to other problems such as amblyopia.)
Some lysosomal storage diseases, in which the patient has an absence or deficiency of an enzyme, leading to inappropriate storage of material in various cells of the body, are associated with corneal clouding. The abnormal accumulation of excess cell products can lead to cells dying and significant medical problems throughout the body, including in the brain and kidneys; these diseases usually lead to death at a young age. Fortunately, these are rare conditions. It’s a challenging diagnosis to make, requiring the assistance of pediatricians and geneticists.
Other problems such as nasolacrimal duct obstruction, conjunctivitis and corneal abrasion or keratitis can cause tearing and light sensitivity that can also mimic childhood glaucoma. Nasolacrimal duct obstruction is very common in babies and young children, but a careful exam will usually clarify the nature of the problem.
Finally, congenital optic nerve anomalies can be misleading; some- look like glaucomatous damage. However, this isn’t too hard to distinguish from true glaucoma, because you won’t find elevated pressure or other signs of glaucoma.
Once a child is brought in to see you, measuring the intraocular pressure is key to supporting or ruling out a diagnosis. Getting the pressure measurement with a toddler—in particular, those from one to three years of age—can be very challenging because they squeeze their eyes shut and fight to prevent you from touching them. If you do force your way in to take a reading, their resistance artificially raises the pressure.
In general, measuring a feeding or sleeping infant is ideal, and a handheld rebound tonometer, such as the iCare tonometer, has been shown to reduce the likelihood of having to
conduct an examination under anesthesia (EUA).2 My experience agrees with that conclusion. Rebound tonometry can often be done while the child is distracted, and it doesn’t re-quire topical anesthetic drops, which burn (making the child very unhappy).
If getting the pressure is impossible, an EUA is standard of care, based on your level of suspicion. However, you’ll have to take into account that general anesthesia tends to cause a decrease in IOP.
Managing the Disease
When treating an adult for glaucoma, the options usually come down to medications, laser trabeculoplasty or surgery. Most ophthalmologists prescribe medications first, although some will consider starting with the laser. (SLT as a primary treatment in adults is supported by some data in the literature.) [For more on this, see the point-counterpoint article in this issue.]
 |
In children, laser trabeculoplasty isn’t helpful. If you develop glaucoma later in life, it means your trabecular meshwork aqueous drainage had been working pretty well, but then it got a little worse, leading to increased IOP. In contrast, if you develop glaucoma early in life, things are usually pretty bad; the drain’s not working well at all. For example, in juvenile open-angle glaucoma, the angle is severely dysfunctional and pressures are really high; small case series haven’t demonstrated any benefit to LT in these patients. (A laser-based treatment is also harder to perform, especially in young children, where it would require anesthesia.)
For many childhood glaucomas, medication is a reasonable primary treatment, moving to surgery if the problem worsens. However, surgery is standard of care for treating primary congenital glaucoma, because it often responds very well to surgery. The effect of medication on PCG is usually in-sufficient.
Another aspect of managing primary congenital glaucoma is that it’s associated with corneal edema. If a child has corneal edema in one eye, the result can be amblyopia, so after you address the glaucoma, you also have to address the weak vision in that eye caused by the visual blockage. Sending good visual information to the brain is essential for developing good vision, especially in the first three months of life when the brain and retina are developing together; if that’s interrupted for any reason during the first 8 to 10 years of life, a child can develop amblyopia.
Amblyopia is potentially reversible, with the use of patching, topical drops such as atropine, and putting the proper spectacle or contact lens correction in front of the eye, but it’s a lot of work. Of course, if you don’t address it in a timely manner, it can become impossible to reverse.
The Anesthesia Dilemma
Relying on surgery to address primary congenital glaucoma—the most common form of childhood glaucoma—leads to an additional challenge: deciding when, and how often, to use anesthesia on your young patient. Putting a child to sleep is necessary for a surgical procedure, but sometimes we even need to do a basic exam under anesthesia because the child won’t cooperate.
Given that anesthesia comes with potential risks, this raises the question of how often you should do a pediatric glaucoma exam under anesthesia. In general, it’s a good idea to avoid putting any patient to sleep too many times, but the particular concern here is that there might be neurodevelopmental effects from early exposure to anesthesia—especially repeated exposure. The problem is, it’s kind of a catch-22. The children that you have to put to sleep already have potential neurodevelopmental issues as a result of the glaucoma; if you don’t put the child to sleep to address the glaucoma, you may not be protecting him from those problems anyway.
The risks associated with anesthesia can be exacerbated by other pre-existing health concerns. For ex-ample, if you’re treating a child with cerebral palsy, the child already has a neurodevelopmental delay. The question is, will anesthesia have any impact on that? Some studies have found evidence suggestive of a negative impact, but didn’t confirm the association.3 One study looked into the impact of a single anesthesia on otherwise healthy children young-er than 36 months and found no statistically significant neurocognitive or behavioral differences later in life compared to children with no anesthesia exposure.4 That’s encouraging.
In my experience, it’s necessary to put children with glaucoma to sleep—especially infants and very young children. Clearly anesthesia is necessary if you need to operate, and there are times when you have to put patients to sleep to examine them. Furthermore, most of the time you’ll need to perform more exams down the road, and those may also require anesthesia. Ultimately, given the concerns surrounding anesthesia, you need to consider each procedure and exam carefully in the context of what’s going on with that patient.
Is Bilateral Surgery Safe?
One of the issues that comes up when treating childhood glaucoma is deciding whether or not to do bilateral surgery (assuming both eyes need treatment). Some doctors do bilateral surgery in the setting of primary congenital glaucoma, which is considered acceptable as a way to avoid additional surgeries. However, I only do bilateral surgery occasionally, for a number of reasons:
• Doing bilateral surgery will require keeping the child asleep for a longer time. This potentially increases the likelihood of unintended consequences. Of course, this is a trade-off; if you avoid doing a bilateral procedure, you’ll have to use anesthesia again later when you perform the second surgery. (Most of the studies that look at bilateral pediatric surgery don’t take into account the frequent need to do exams under anesthesia later.) Nevertheless, it may be safer to use anesthesia for shorter periods of time. Also, if you do bilateral surgery, you lose several advantages that come with performing sequential surgery:
• If you just operate on one eye, you can see the result before operating on the second eye. If a problem arises, you can take steps to ensure it doesn’t occur during the second surgery.
• Doing one eye right after the other opens the door to cross-contamination. One of the risks associated with bilateral surgery is the possibility that any issue with one eye—such as endophthalmitis—could end up also affecting the other eye, despite your best efforts to prevent it.
• If there’s a complication, you don’t want both eyes disabled. If you do surgery in both eyes and have complications in both eyes, then the child is visually impaired in both eyes until the complication is resolved. That might impact the child’s function and development.
There are situations in which I’d be more likely to consider performing bilateral surgery—particularly if the child has short-term risks tied to anesthesia, such as asthma or heart problems. Also, some patients are refractory; they’re not responding to treatment, and/or their visual potential is very poor or nil. Putting those patients to sleep frequently is likely to increase the risk of a medical problem, including possible neurodevelopmental issues. You shouldn’t keep putting kids to sleep if they’re doing poorly and the odds that you’re helping them are low.
Of course, any risk associated with using anesthesia on a young patient is small. The reality is, if the anesthesia is applied and monitored properly, and the child doesn’t have any other conditions that would put him or her at risk, the likelihood of any adverse outcome is very low.
In the General Clinic
In reality, many comprehensive ophthalmologists don’t see young children. In addition, most children who have a health issue of any kind, including vision, are likely to be taken to a pediatrician. From there, they may end up in the office of a pediatric ophthalmologist or an optometrist with pediatric expertise. The moment they suspect glaucoma, most will send the child to someone with pediatric glaucoma expertise.
Certainly, if you do see a young child and you suspect glaucoma, a referral is appropriate. A child with primary congenital glaucoma, whose cornea looks cloudy or edematous, is an ASAP referral. Older children who may be at risk are usually a less-urgent referral, unless the pressure is very high or the optic nerve shows a lot of damage.
The most common thing a comprehensive ophthalmologist is likely to see is a child with an enlarged cup-to-disk ratio. Many of these children don’t have glaucoma; they simply have physiologic cupping, but that’s a common source of referral. With an older, more cooperative child, you can get a pressure, an OCT and maybe even a visual field if you need to. Children with abnormal testing or elevated IOP should be referred for further evaluation by someone with pediatric glaucoma expertise. REVIEW
Dr. Beck has received grant support from Allergan. He reports no financial interest in any product mentioned in this article.
1. Freedman SF, Lynn MJ, Beck AD, et al. Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol 2015;133:8:907-14.
2. Grigorian F, Grigorian AP, Olitsky SE. The use of the iCare tonometer reduced the need for anesthesia to measure intraocular pressure in children. J AAPOS 2012;16:6:508-10.
3. Chang TC, Cavuoto KM. Anesthesia considerations in pediatric glaucoma management. Curr Opin Ophthalmol 2014;25:2:118-21.
4. Sun LS, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 2016;315:21:2312-20.