All of these barriers, however, can be surmounted if you accomplish five things:
• educate your patients about glaucoma;
• teach proper instillation;
• increase the time the medication remains on the eye;
• improve drug penetration; and
• choose the most efficacious regimen for each patient.
Here, I’d like to share 14 specific steps you can take that will help ensure that your patients get the most out of their topical medications. (Note: In this day and age, when we’re being asked to take care of more patients with fewer resources, it may not be easy to personally execute all of the strategies listed below with your patients. However, well-trained technicians should be able to do much of this for you.)
1. Educate the patient. As everyone knows, lack of patient adherence to a prescribed protocol is a prime cause of reduced efficacy. One reason for lack of adherence is that many patients don’t really understand the value of the medication, especially since glaucoma is usually painless and doesn’t elicit symptoms until a lot of damage has already been done. At the end of the day, a patient who fully understands the disease and the importance of taking the drops is far more likely to follow instructions. Studies have confirmed this.1,2
For patient education to be effective, you need to:
• Maintain a good doctor-patient relationship. Without this, the patient will be far less interested in taking your advice.
• Make sure the patient understands what glaucoma is and how it affects vision. As noted, patients often have a poor grasp of the reasons they need to be concerned.
• Educate the patient about the medication, including how it works, how it will benefit the patient when taken properly and any side effects it may have. The last item is important because if you give someone a medication that has a side effect without mentioning it, the patient may sim-ply stop using the drop when he encounters the side effect.
It may help to provide the patient with pamphlets and brochures containing much of this information. However, this approach may be best when used to supplement face-to-face education in the office.
2. Ask patients open-ended questions about their medication use. This will often get them to reveal problems they’re having that are affecting their compliance. For instance, you might ask, “How often do you miss taking your medications?” Or, “Are you having any problems with your medications?” The patient is more likely to admit problems if you take the time to ask.
3. Show patients evidence of their disease severity and/or progression. Showing patients how their nerve fiber layer has changed on optical coherence tomography since the previous visit, for example, can go a long way toward getting them to take the disease seriously. You might
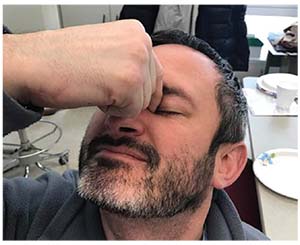 |
| Manual punctal occlusion increases the amount of drug getting into the eye and decreases systemic absorption. |
4. Watch the patient instill a drop. Many ophthalmologists don’t take the time to do this, but it may be the crux of the matter. You’ll almost always find several things that patients are not doing optimally to make the medication work. For instance, some patients actually apply the drops to the outside of the eye! By watching the patient instill a drop, you may catch a problem and be able to address it right up front, preventing all of your efforts from being wasted.
5. Show the patient proper instillation technique. This is the other half of getting patients to show you their instillation technique: teaching them to do it right. Showing them your technique of choice while they’re in the office is ideal, but you can also refer patients to videos on YouTube. There are thousands of videos showing how to instill drops; you can find one that matches your technique and ask patients to watch it several times at home. Better yet, get them to watch it in the office (so you can be sure they’ve seen it).
After they’ve seen the video, of course, you should check to see what the patient actually ends up doing. If you stay on the case until the patient learns to correctly instill the drops, you’ll go a long way towards eliminating a major cause of treatment failure.
6. Teach patients to avoid the washout effect. Even if a patient’s technique is successfully getting drops onto the eye, when more than one drop is being used, the washout effect potentially caused by the second drop can undo the potential value of the first drop.3 I tell patients that when they instill a topical medication in the eye, they should wait at least five minutes before instilling the second drop so that they don’t wash out the first medication.
7. Consider prescribing a combination medication. Combining two medications into one bottle is an excellent way to avoid the potential problems caused by having to manage multiple medications—including the problem of a second drop washing out the first.4
8. Teach the patient to perform manual punctal occlusion. Punctal plugs prevent a drug placed on the eye from draining down into the throat as quickly, thus increasing the residence time, penetration and efficacy of the drug.5 Fortunately, patients can duplicate this effect in a temporary manner by blocking the lower puncta with the fingers (usually the index finger and thumb, on either side of the nose—see image, left), which has also been shown to be effective.6 Furthermore, blocking the flow of medication into the throat decreases the systemic side effects of the medication.
Closing the eye after instilling the medication is another technique that’s also recommended by many doctors, but I believe that manual punctal occlusion is the most effective strategy. (In any case, the patient may want to close his or her eyes whenever fingers are placed that close to the eye.)
9. Consider prescribing a gel. Doctors sometimes forget that many medications are available in gel form. Increased viscosity leads to increased time on the cornea, more sustained released and improved bioavailability, allowing the drug to be more effective.7
A second benefit in some cases is that the gel version of a drug may need to be used less often. For example, the drop version of timolol is generally used twice a day, whereas the gel version only needs a single application per day. The downside of using a gel, of course, is that there’s some transient blurriness associated with it. Many patients, however, won’t find this objectionable, especially if it reduces the number of applications per day.
10. Teach the patient to induce a temporary dry-eye state. As noted earlier, the tear film is one of the eye’s natural barriers, helping to prevent unwanted substances from getting inside the eye. But in the case of a glaucoma medication, we want to get past as many barriers as possible to ensure that the medication has a chance to help the patient. As it turns out, there’s a simple way to temporarily minimize the tear-film barrier and let more medication through.
If your patient resists the urge to blink—perhaps by engaging in a staring contest for 10 seconds—this creates a temporary dry-eye state. It causes a decrease in the quantity and quality of the tear film and an increase in tear-film breakup time, minimizing the tear-film barrier. If a drop is applied with the tear-film barrier reduced, more drug is absorbed into the cornea. This was demonstrated in one study in which researchers created a dry-eye state in one eye while allowing the other eye to blink normally, as a control. They then instilled either pilocarpine or phenylephrine into both eyes and measured how long it took for the pupils to constrict or dilate. They found that the medications entered into the cornea significantly faster in the eye that had the dry-eye state.8
11. When possible, prescribe prodrugs. The other barrier that keeps unwanted chemicals out of the eye is the cornea itself. One reason the cornea is so effective as a barrier is that it’s made up of a lipophilic epithelium and a hydrophilic stroma. That makes it very difficult for any medications to penetrate all the way through the cornea, whether the medication is hydrophilic or lipophilic; if one layer doesn’t block the drug, the other layer will.
Nevertheless, some drugs are able to make it past this obstacle by changing form as they go through the cornea. These are referred to as prodrugs. These molecules have a lipophilic segment that initially allows them to penetrate into the epithelium. Then, as they go into the stroma, they undergo hydrolysis by esterases, which causes them to become hydrophilic and allows them to get through the stroma. This results in the slow release of significant concentrations into the aqueous humor.9,10
All of the prostaglandins except for Lumigan—including Travatan and Xalatan—are prodrugs.
12. Choose a second medication wisely. If it’s necessary to add a second drop to the patient’s regimen, it’s important to choose one that will be most effective in conjunction with the first drop. If I’m adding a second-line agent to the patient’s regimen, I generally choose a drop that works via a different mechanism. Prostaglandins, for example, work by increasing uveoscleral outflow. Therefore, if I’ve started with a prostaglandin and the patient had a good response but the pressure still isn’t low enough, my second-line agent would be something that decreases aqueous humor production, such as a carbonic anhydrase inhibitor or a beta-blocker. The combination of two different mechanisms tends to be more effective.
In addition, studies have demonstrated that some of these drugs do better than others as a second-line agent, in terms of the percentage of patients that show a ≥10 percent extra pressure reduction when the second drug is added. One study found that 84 percent achieved this reduction when a CAI was added; 61 percent achieved this when a beta blocker was added; and 44 percent achieved this when an alpha agonist was added.11 For that reason, I favor CAIs as my go-to second-line agent.
13. Check for systemic drugs being used by the patient. Patients on systemic equivalents to a topical drop may not respond as well to the drops.12 For example, if the patient is on a systemic beta blocker for a heart condition or blood pressure problem, he might not have as much of an IOP response to a topical beta blocker. Similarly, systemic CAIs are sometimes used for blood pressure control or conditions such as pseudotumor cerebri to lower intracranial pressure; the same principle applies.
14. Remember that you can increase the effect of some medications by increasing the frequency of the drops. Although there’s nothing to gain from increasing the frequency of drugs such as prostaglandins or beta blockers, studies have shown that if you increase your CAI or alpha agonist dosage from two times a day to three times a day, you may get another point or two of IOP lowering.13 This also works with a combination drop such as Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension). REVIEW
Dr. Patrianakos is chair of ophthalmology for the Cook County Health and Hospitals System. He has no financial interest in any product mentioned in this article.
1. Carpenter DM, Blalock SJ, Sayner R, et al. Communication Predicts Medication Self-Efficacy in Glaucoma Patients. Optom Vis Sci 2016;93:7:731-7.
2. Han SR. Patient-centered communication to assess and enhance patient adherence to glaucoma medication. Ophthalmology 2009;116(11 Suppl):S37-42.
3. Pfeiffer N, TATS (Travatan Adjunctive Treatment Study) group. Timolol versus brinzolamide added to travoprost in glaucoma or ocular hypertension. Graefes Arch Clin Exp Ophthalmol 2011;249:7:1065-71.
4. Inoue K, Okayama R, Higa R, et al. Ocular hypotensive effects and safety over 3 months of switching from an unfixed combination to latanoprost 0.005%/timolol maleate 0.5% fixed combination. J Ocul Pharmacol Ther 2011;27:6:581-7.
5. Opitz DL, Tung S, Jang US, Park JJ. Silicone punctal plugs as an adjunctive therapy for open-angle glaucoma and ocular hypertension. Clin Exp Optom 2011;94:5:438-42.
6. Zimmerman TJ, Kooner KS, Kandarakis AS, Ziegler LP. Improving the therapeutic index of topically applied ocular drugs. Arch Ophthalmol 1984;102:4:551-3.
7. Bourlais CL, Acar L, Zia H, et al. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res 1998;17:1:33-58.
8. Abelson MB, Ousler GW. Instillation of ophthalmic agents after tear film break-up time to enhance treatment effect. Invest Ophthalmol Vis Sci 2001;42:4:S176.
9. Barot M, Bagui M, Gokulgandhi MR, Mitra AK. Prodrug strategies in ocular drug delivery. Med Chem 2012;8:4:753-68.
10. Dey S, Anand BS, Patel J, Mitra AK. Transporters/receptors in the anterior chamber: pathways to explore ocular drug delivery strategies. Expert Opin Biol Ther 2003;3:1:23-44.
11. Reis R, Queiroz CF, Santos LC, Avila MP, Magacho L. A randomized, investigator-masked, 4-week study comparing timolol maleate 0.5%, brinzolamide 1%, and brimonidine tartrate 0.2% as adjunctive therapies to travoprost 0.004% in adults with primary open-angle glaucoma or ocular hypertension. Clin Ther 2006;28:4:552-9.
12. Schuman JS. Effects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Brimonidine Study Groups 1 and 2. Ophthalmology 2000;107:6:1171-7.
13. Konstas AG, Stewart WC, Topouzis F, et al. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% in primary open-angle glaucoma. Am J Ophthalmol 2001;131:6:729-33.



