Traditional therapies for amblyopia have limited effectiveness, are uncomfortable and can be hard on both the patients and their families. For these reasons, both clinicians and researchers alike have been working on better treatments that help avoid the drawbacks of past therapies.
Today, advanced technology is allowing the development of new ways to diagnose and treat amblyopia. Here are some new technologies that are showing promise.
The Nature of the Problem
Amblyopia can be the result of many different factors. Conditions causing it can include myopia, hyperopia, astigmatism, strabismus or cloudiness in the crystalline lens. The vision in the underused eye then gets worse as the brain suppresses its visual input. Left untreated, this can lead to long-term deficits in vision.
Amblyopia is currently the leading cause of preventable monocular vision loss, affecting about 3 percent of all children in the United States.1-3 In addition to impaired perception in the underused eye, amblyopia causes a loss of stereopsis and depth perception,4 a reading speed about 25 percent slower than other children,5 impaired motor skills and loss of dexterity,6-11 lower self-confidence12-14 and lower self-reported quality of life.15
The Traditional Approach
On the positive side, studies have demonstrated that repeated early screening of children for amblyopia dramatically decreases its prevalence (compared to waiting until children start school to begin screening).16-18 Also, studies have also shown that initiating therapy before age 3 causes a 70-percent reduction in amblyopia prevalence.16-18
Conventional treatment has included eye patching or blurring atropine eye drops, intended to minimize the use of the stronger eye and force the child to use the weaker eye. Drawbacks include that these approaches don’t train the eyes to work together; they produce notoriously poor compliance; and they can stigmatize the child among his or her peers. In addition, these approaches fail to restore normal visual function in a significant percentage of children.
Furthermore, traditional means of diagnosing amblyopia have relied on detecting vision problems that sometimes lead to amblyopia, rather than detecting amblyopia itself. Data suggests that one in every five children has at least one risk factor for amblyopia, but only one in 40 children actually has amblyopia. As a result, only one out of every eight children referred for further evaluation—based on having a risk factor—actually has amblyopia. (That’s a false positive rate of about 87 percent.) This results in very high rates of unnecessary referrals for treatment.
Eileen E. Birch, PhD, an adjunct professor of ophthalmology at the University of Texas Southwestern Medical Center, and director of the Crystal Charity Ball Pediatric Vision Laboratory, part of the Retina Foundation of the Southwest, points out that most pediatricians and vision screening programs still rely on visual acuity charts to test for amblyopia. “One problem with that approach is that only a third of 3-year-old children and half of 4-year-old children can complete the test,” she says. “Sometimes automated tests are used, but they’re also less than ideal because they detect hyperopia and anisometropia instead of amblyopia. Only a small fraction of those testing positive for hyperopia or anisometropia actually have amblyopia. Many pediatricians don’t even attempt to screen for amblyopia because the false-positive rate is so high.”
New Tech for Diagnosis
The Blinq Pediatric Vision Scanner (Rebion, Boston) is a handheld device that uses a laser probe to detect abnormal bifoveal fixation, an early sign of amblyopia. This technique, called neural performance scanning, has been cleared by the FDA for microstrabismus and amblyopia screening.
The Blinq device is held 14 inches from the child’s eyes. The child fixates on a smiley face while the device scans both retinas in just over two seconds; it can detect a misalignment between the foveas as small as one degree. The device calculates a binocularity score and gives a “pass” or “refer” signal after the two-second test, so there’s no need to interpret numerical data.
 |
|
The Blinq Pediatric Vision Scanner (Rebion) detects abnormal bifoveal fixation as small as one degree. The device provides a “pass” or “refer” signal after testing. Unlike traditional amblyopia screening tests, Blinq has a low false-positive rate, the company says. (Image courtesy of Rebion.) |
One major selling point for the Blinq device is its low false positive rate—especially compared to previous methods used to scan for amblyopia, such as visual acuity charts looking for refractive error. Using Blinq, false positives from testing young children have been 15 percent or lower in some clinical studies. For example, in one clinical trial testing the previous iteration of the device (the Pediatric Vision Scanner), 300 children 2 to 5 years of age, with no known eye problems, were scanned.19 Six children who had been determined by a specialist’s exam to have amblyopia were all correctly identified by the device. That study found a false positive rate of 15 percent, using this earlier iteration of the device. (That rate dropped to 9 percent when uncooperative children were omitted from the dataset.)
Another prospective study published in 2021 using the new Blinq device evaluated 193 individuals ranging in age from 1 to 20 years.20 In this population, sensitivity was 100 percent and specificity was 91 percent. In a subanalysis of children aged 2 to 8 years in this study (n=92), sensitivity was 100 percent and specificity was 89 percent. However, a study conducted in Belgium involving 101 children between ages 2 and 8, published this spring, found a specificity of only 73.1 percent.21
Dr. Birch explains that detecting amblyopia directly is what makes Blinq so promising. “Because of the unique architecture of the Henle fibers in the fovea, retinal birefringence scanning using polarized light can detect whether fixation is steady or eccentric,” she says. “Steady fixation causes frequency doubling in the reflected signal, with a dominant frequency of 200 Hz in the returning light. Unsteady fixation, caused by amblyopia, produces little or no birefringence, with little or no 200-Hz frequency light in the reflected signal.”
Dr. Birch says that her group conducted two studies in which 400 preschool children (2 to 6 years of age) were screened using a birefringence scanner at three pediatric care practices.22 “We compared our results to those of a gold-standard comprehensive exam done by a pediatric ophthalmologist,” she says. “Scanning with birefringence had 97- percent sensitivity and 90-percent specificity. This was a significantly lower false-positive rate than that produced by the automated scanners that check for risk factors only.”
High-tech Eyeglasses
CureSight, from NovaSight (Airport City, Israel) is a binocular, eye-tracking-based, digital vision-treatment system designed for patients ages 4 to 9 suffering from amblyopia. The child watches any streamed video content of choice through red and blue glasses. CureSight blurs the visual center of the image that’s being shown to the stronger eye using real-time image processing, based on the momentary gaze position being captured by the eye tracker. The company says that the blurred center of vision of the stronger eye forces the brain to complete the image using the details available to the amblyopic eye. This gets the patient’s visual system in the habit of relying more on the weaker eye, while training the two eyes to work together. CureSight is designed to be used at home, under the remote supervision of an eye-care provider and NovaSight’s Monitoring Center.
This spring, NovaSight announced data from a multicenter, randomized, controlled trial of the system. The study involved 103 kids aged 4 to 9, done at six medical centers; it compared the visual improvement achieved by CureSight treatment to that produced by eye patching. Findings included:
- The CureSight system was shown to be noninferior to eye patching for amblyopia treatment.
- BCVA improvement at week 16 was larger in the CureSight group than in the patching control group.
- No serious adverse events were observed in either treatment arm.
- Mean adherence to the CureSight protocol, as measured by the CureSight’s eye tracking system, was 93 percent at week 16 (n=43). (Adherence to patching is notoriously limited.)
- Ninety-three percent of parents reported that they’re likely to choose the CureSight digital treatment over patching.
“During the study, binocular visual acuity and stereo acuity improved significantly in both the CureSight and patching groups,” says Tamara Wygnanski-Jaffe, MD, head of the Pediatric Ophthalmology and Strabismus Service at The Goldschleger Eye Institute of the Sheba Medical Center in Israel. (She participated in the CureSight clinical trial and is an unpaid member of the NovaSight advisory board.) “Distance visual acuity in the amblyopic eye improved similarly in both groups. However, vision improvement in eyes treated with patching plateaued at week 12, while children treated with CureSight kept improving through week 16.”
 |
|
CureSight (NovaSight) lets a child watch any video content through special glasses. Using eye tracking to monitor the child’s gaze, the system blurs the visual center of the image being seen by the stronger eye, forcing the brain to incorporate information from the weaker eye. (Image courtesy of NovaSight.) |
Dr. Wygnanski-Jaffe points out that patching, although effective, has several inherent shortcomings. “Patching only achieves low adherence, in part because it’s uncomfortable,” she notes. “In contrast, binocular therapy with CureSight takes place in the patient’s home, with an unlimited variety of streamed content chosen by the child or parents. Furthermore, unlike some binocular treatments for amblyopia that include VR headsets and tablet games, CureSight isn’t limited by interpupillary distance or fitting issues, and it doesn’t require the use of cumbersome goggles.”
Dr. Wygnanski-Jaffe points out that CureSight is essentially a remote patient monitoring system. “Prescribing physicians have access to CureSight’s dedicated cloud platform, which allows them to monitor patients’ progress remotely,” she explains. “Furthermore, a dedicated monitoring center observes the progress of each patient’s treatment and the extent of compliance. If it detects low compliance, it contacts the patient’s guardians to ensure that the child proceeds with the treatment as prescribed.”
Dr. Wygnanski-Jaffe says the company is planning to increase the device’s flexibility to allow it to treat adult amblyopes. “A small feasibility study has shown promising results, and NovaSight is planning to initiate a larger study,” she explains. “Meanwhile, the company is working on developing the second generation of the CureSight device, which will be designed to treat cases of strabismic amblyopia by shifting the two images in the screen plane to cope with the momentary deviation angle between the eyes.”
The CureSight system currently has the CE mark and has recently applied for a 510K FDA approval.
Virtual Reality Goggles
 |
|
Luminopia One (Luminopia) allows a child to watch any of a library of popular kid-friendly TV shows and movies. The visuals have been processed to require the brain to combine the images during viewing. (Image courtesy of Luminopia.) |
Luminopia One (from Luminopia in Cambridge, Massachusetts) is a new prescription digital therapy software for kids 4 to 7 years old who have amblyopia associated with anisometropia and/or mild strabismus. Luminopia One works by visually modifying any of a library of popular TV shows and movies for a young patient to watch through a virtual reality headset. The software alters the images seen by each eye in a way that requires the brain to combine the images, strengthening visual processing, promoting increased use of the weaker eye and encouraging the eyes to work together.
The system was developed in collaboration with leading clinicians and researchers at Boston Children’s Hospital and MIT. Available videos for use with Luminopia One include more than 700 hours of popular and educational content from media companies such as Sesame Workshop and Nickelodeon. Meanwhile, a cloud-based platform tracks the child’s usage, monitoring adherence to treatment. As a result, parents can control what the child is watching and track how their child is doing.
The company has conducted multiple clinical trials of Luminopia One working with hospitals and research institutions across the United States, including a prospective, randomized, controlled Phase III trial. In that trial, 105 children with amblyopia aged 4 to 7 were enrolled at 21 sites, randomized 1:1 to treatment or a control group.23 Those in the treatment group used the system at home one hour a day, six days a week for 12 weeks (the protocol recommended for standard patients).
At 12 weeks, amblyopic eye visual acuity improved by 1.8 lines (95% CI, 1.4-2.3 lines; n = 45) in the treatment group; in the control group visual acuity improved by 0.8 lines (95% CI, 0.4-1.3 lines; n = 45), a significant difference (p=0.0011). Eighty-four percent of children in the treatment group had previously undergone treatment with patching or atropine drops, but the treatment efficacy of Luminopia One was still significant in this subgroup. No serious adverse events were reported. (The pilot studies using this technology have also shown efficacy in older children and adolescents, for whom eye-patching and atropine eye drops are largely ineffective.)
“Until now, clinically available amblyopia treatments have involved blocking or blurring the stronger eye to make the brain pay more attention to the amblyopic, or ‘lazy’ eye,” notes David G. Hunter, MD, PhD, ophthalmologist-in-chief at Boston Children’s Hospital and professor and vice chair of ophthalmology at Harvard Medical School, and a scientific advisor to Luminopia. “Unfortunately, neither of these approaches actively promotes the two eyes working together. Instead of blocking one eye to favor the other, Luminopia One lets the child watch movies that are modified so the amblyopic eye sees some parts of the image while the fellow eye sees the other parts. The brain has to put the pieces together to create complete images.”
According to Dr. Hunter, Luminopia One has several helpful features, including:
- The Luminopia One device doesn’t place colored filters over the eyes. This means that both the amblyopic eye and the fellow eye see the full spectrum of color.
- Luminopia One’s headset-based approach creates a controlled environment free of distractions for the child.
- The virtual reality headset presents images at optical infinity, avoiding any issues related to accommodation (which can be limited in amblyopic eyes) or convergence.
“Most kids love to use Luminopia One, since their ‘assignment’ is to watch whatever movie they want to watch for an hour per day,” Dr. Hunter notes. “In one case, a child brought the device to school for Show and Tell. This is in contrast to the social stigma and bullying that can hinder eye-patching compliance.”
Dr. Hunter points out a few things of interest that emerged from the clinical trials. “Most of the patients in these trials had already maxed out on patching, yet improvement resumed once they started treating with Luminopia One,” he says. “Furthermore, these gains were achieved with treatment ‘doses’ much lower than those used for patching: one hour per day, six days a week for Luminopia One, versus two to six hours per day—up to 12 hours per day in some cases—seven days a week for eye patching. I don’t think any other digital treatment has shown this kind of efficacy.”
What about limitations? “The VR goggles can be heavy for the youngest children,” Dr. Hunter acknowledges. “However, Luminopia has updated the design so that kids can lie down while watching the programs. Also, treatment using Luminopia One is likely to cost more than patching. However, because it’s been proven to be an effective therapy, I expect it will ultimately be covered by insurance. Meanwhile, for the physician, it should be as simple as prescribing a drug or drop through your electronic health records system, or even using a paper prescription.”
Luminopia One achieved FDA de novo premarket approval for prescription use last fall; the Luminopia One product launch is scheduled for later this year. Meanwhile, the company is developing a series of other devices intended to treat multiple neuro-visual disorders using similar technology, and negotiating with regional and national insurers to obtain coverage for Luminopia One as a pharmacy benefit.
Hope for Amblyopic Adults?
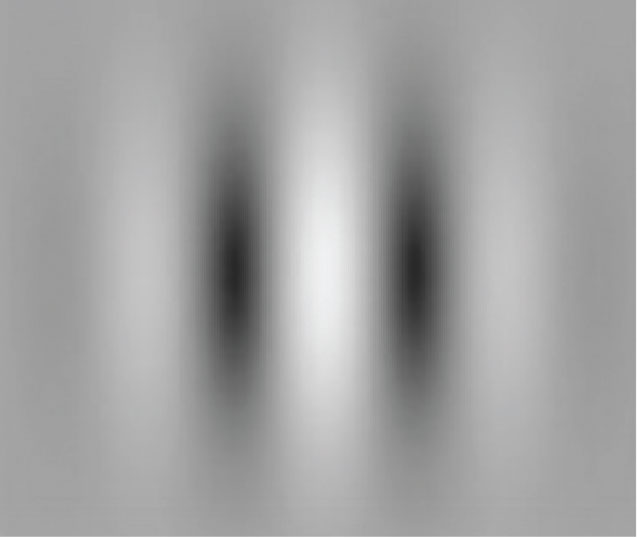 |
|
RevitalVision, FDA-approved for patients 9 years of age and older, gives the patient visual exercises using Gabor patches that gradually improve the brain’s visual processing, increasing visual acuity, contrast sensitivity and stereo acuity, the company says. (Image courtesy of RevitalVision.) |
Other recent treatment approaches have been aimed at adults with amblyopia. (Conventional eye patching is generally ineffective for adults.) The only one currently approved by the FDA is RevitalVision, approved for use by patients age 9 and older. This is a prescription “vision-training” software that uses visual exercises involving Gabor patches to stimulate the brain’s visual cortex. The company says that it takes advantage of brain plasticity to improve the visual cortex’s processing of incoming visual data in amblyopic adults. The company notes that in contrast to most therapies, this is aimed at the visual cortex rather than the eyes.
Treatment involves 40 training sessions done on a home computer, completed over a period of three months with three to four sessions per week that last 30 minutes on average. The sessions are customized to accommodate the visual ability and working speed of the patient, with results remotely monitored by an eye-care professional.
According to the company, randomized, controlled clinical studies have shown that adults undergoing treatment have an average improvement of 2.5 lines on a visual acuity chart, a 100-percent improvement in contrast sensitivity and significant improvement in stereo acuity. The company also says the technology has been shown to improve vision in other conditions such as congenital nystagmus, and helps patients who are having difficulty neuroadapting after cataract surgery.
1. Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in White and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009;116:2128–34:e1–2.
2. Giordano L, Friedman DS, Repka MX, et al. Prevalence of refractive error among preschool children in an urban population: The Baltimore Pediatric Eye Disease Study. Ophthalmology 2009;116:739–46:e1–4.
3. Multi-Ethnic Pediatric Eye Disease Study. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months in the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology 2008;115:1229–36:e1.
4. Birch EE, Kelly KR, Giaschi DE. Fellow eye deficits in amblyopia. J Binocul Vis Ocul Motil 2019;69:116–125.
5. Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 2015;19:515–520.
6. Kelly KR, Morale SE, Beauchamp CL, et al. Factors associated with impaired motor skills in strabismic and anisometropic children. Invest Ophalmol Vis Sci 2020;61:43.
7. Webber A. The functional impact of amblyopia. Clin Expl Optom 2018;101:443–450.
8. O’Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Invest Ophalmol Vis Sci 2010;51:2019–2023.
9. Grant S, Suttle C, Melmoth DR, et al. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest Ophalmol Vis Sci 2014;55:5687–5701.
10. Niechwiej-Szwedo E, Goltz HC, Colpa L, et al. Effects of reduced acuity and stereo acuity on saccades and reaching movements in adults with amblyopia and strabismus. Invest Ophthalmol Vis Sci 2017;58:914–921.
11. Webber A, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008;49:594–603.
12. Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol 2019;137:167–173.
13. Webber A, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci 2008;85:1074–1081.
14. Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception in children aged 3 to 7 years with amblyopia and its association with deficits in vision and fine motor skills. JAMA Ophthalmol 2019;137:499–506.
15. Hatt SR, Leske DA, Castaneda YS, et al. Understanding the impact of residual amblyopia on functional vision and eye-related quality of life using the PedEyeQ. Am J Ophthalmol 2020;218:173–181.
16. Williams C, Northstone K, Howard M, et al. Prevalence and risk factors for common vision problems in children: Data from the ALSPAC study. Br J Ophthalmol 2008;92:959-64.
17. Williams C, Harrad R. Amblyopia: Contemporary clinical issues. Strabismus 2006;14:43-50.
18. Williams C, Harrad RA, et al. Screening for amblyopia in preschool children: Results of a population-based, randomised controlled trial. Ophthalmic Epidemiol 2001;8:279-95.
19. Shah SS, Jimenez JJ, Rozema EJ, et al. Validation of the Pediatric Vision Scanner in a normal preschool population. JAAPOS 2021;25:4:216.e1-216.e4.
20. Bosque LE, Yamarino CR, Salcedo N, et al. Evaluation of the Blinq Vision Scanner for detection of amblyopia and strabismus. J AAPOS 2021;25:4:214.e1-214.e7.
21. Devlieger A, Youssfi A, Cordonnier M. Evaluation of the Blinq Vision Screener in the detection of amblyopia and strabismus in children. Transl Vis Sci Technol 2022;11:4:10.
22. Jost RM, Stager Jr D, Dao L, et al. High specificity of the Pediatric Vision Scanner in a private practice pediatric primary care setting. Journal of AAPOS 2015;19:521-5.
23. Xiao S, Angjeli A, Wu HC, et al. Randomized controlled trial of a dichoptic digital therapeutic for amblyopia. Ophthalmology 2022;129:1:77-85.




