 |
|
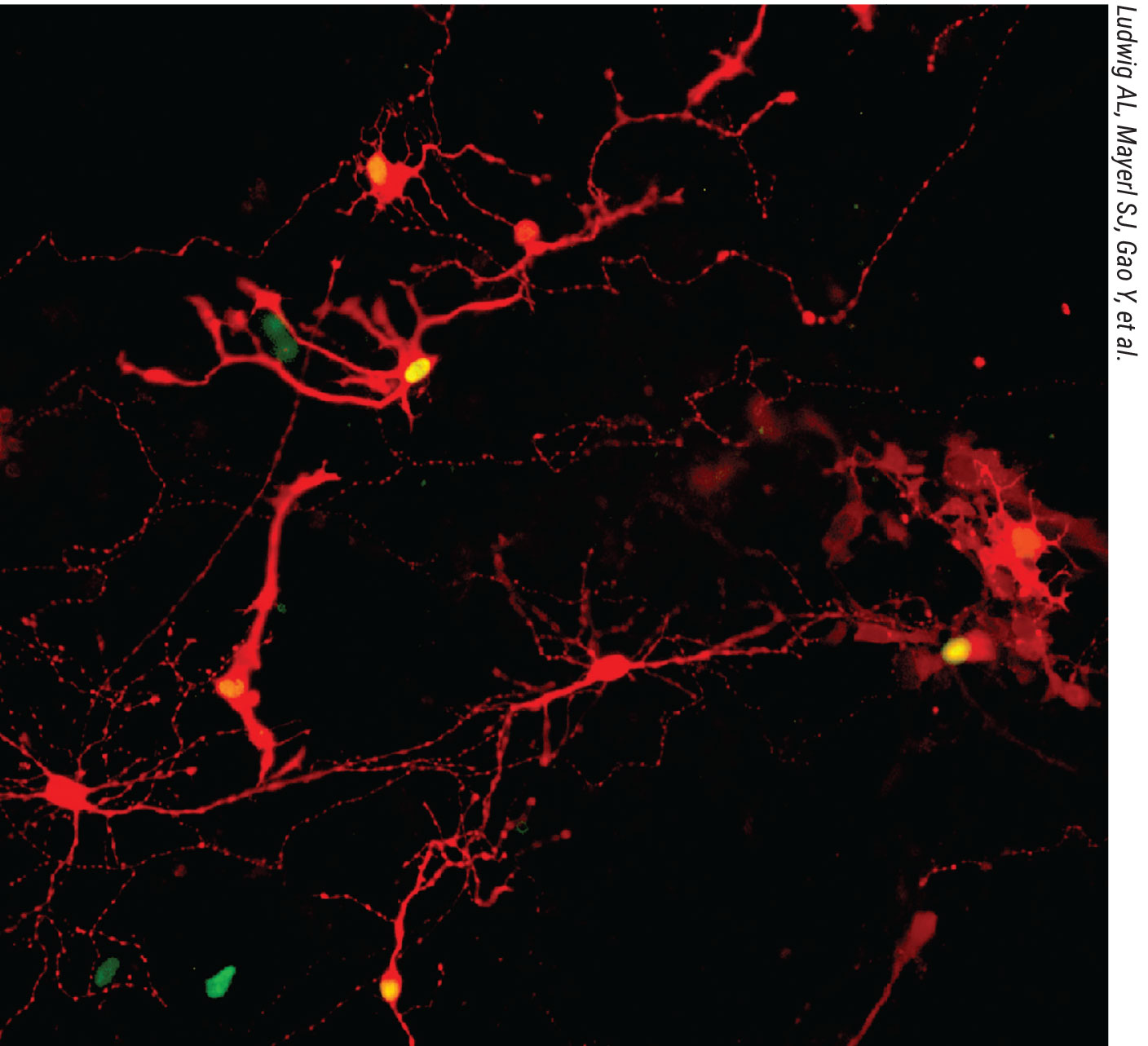
Human pluripotent stem cell-derived retinal neurons were dissociated from retinal organoids and subjected to a viral tracing assay to assess the capacity for re-formation of synaptic connections. Post-synaptic retinal cells possess red cytoplasm with green nuclei whereas pre-synaptic traced retinal cells have red cytoplasm only. (Image courtesy of Ludwig AL, Mayerl SJ, Gao Y, et al.) |
The human body’s functions rely on communication from one system to another, and there’s been ongoing research on how to replicate these intricate systems in a lab. A newly published study in the Proceedings of the National Academy of Sciences has made a landmark discovery with retinal organoids, reproducing synaptic connections in cultured retinal neurons.1
Researchers from the University of Wisconsin-Madison wanted to know if photoreceptors and retinal ganglion cells, once separated from the organoid, could extend their axons and make a connection with other cells nearby. Using a rabies virus tracing assay, researchers found that the photoreceptors did, in fact, reach out and create a synapse.
This was the third phase of two precursor studies conducted at UW-Madison which showed how lab-grown photoreceptors responded to wavelengths and intensities of light2 and then developed axons.3
The next logical question is how this discovery could impact treatment for patients with retinal diseases, such as retinitis pigmentosa and age-related macular degeneration.
David Gamm, a UW-Madison ophthalmology professor, is the director of the McPherson Eye Research Institute where the organoids were developed. He says this research establishes a capability that, if recapitulated in a human patient, it could provide a means for improving vision or providing meaningful improvement in vision for patients who have lost sight due to photoreceptor-based diseases.
“We looked at any cell types that could make these connections, and it turns out that photoreceptors were the most successful, and retinal ganglion cells were the next most successful,” Dr. Gamm says. However, he says, because ganglion cells have to reach through the optic nerve and into the brain, it’s a more difficult task to achieve. “Just because we can show that these connections can be made, doesn’t mean that there aren’t a lot of challenges that are still in front of us to replace these individual cell types. We’re focusing on photoreceptors and trying to see if we can replace some photoreceptors in patients and improve vision to a meaningful degree.”
Dr. Gamm warns it’s important to right-size expectations. “I think people who are in this field are very savvy and understand that these are all step-wise advancements and putting it all together requires a clinical trial and a lot of factors to come together,” he says. “Until we do the clinical trials, we’ll never know, so it’s important to look at things like this particular study and many others that have been published and recognize they’re positive findings and that the puzzle pieces are there and they have the potential to fit together. We’re hoping to make a safe and thoughtful step forward and then improve upon it thereafter.”
1. Ludwig AL, Mayerl SJ, Gao Y, Banghart M, Bacig C, Fernandez Zepeda MA, Zhao X, Gamm DM. Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures. Proc Natl Acad Sci USA 2023;10;120:2:e2213418120.
2. Saha A, Capowski E, Fernandez Zepeda MA, Nelson EC, Gamm DM, Sinha R. Cone photoreceptors in human stem cell-derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell 2022;3:29:3:460-471.e3.
3. Rempel SK, Welch MJ, Ludwig AL, Phillips MJ, Kancherla Y, Zack DJ, Gamm DM, Gómez TM. Human photoreceptors switch from autonomous axon extension to cell-mediated process pulling during synaptic marker redistribution. Cell Rep 2022;17:39:7:110827.
Industry news
Bausch + Lomb Acquires AcuFocus Bausch + Lomb and AcuFocus announced an affiliate of Bausch + Lomb acquired
Glaukos Announces Results for iDose TR Trial Glaukos announced results for a prospective, multicenter clinical trial designed to evaluate the safety of the surgical exchange procedure for iDose TR (travoprost intraocular implant) in subjects who had previously been administered an iDose TR in the Phase IIb clinical trial. The company says that results demonstrated a second administration of iDose TR and removal of the original iDose TR implant was safe and well-tolerated.
Neurophth Receives FDA IND Clearance for AAV-ND1 Neurophth Therapeutics received FDA clearance for its investigational new drug application for NFS-02 (rAAV2-ND1), an in vivo gene replacement therapy to treat Leber hereditary optic neuropathy associated with the ND1 mutation.
Melt Achieves Primary Sedation Endpoint in Phase II Study Melt Pharmaceuticals announced positive topline results of its Phase II efficacy and safety study for lead product candidate, MELT-300, a sublingual, needle- and opioid-free patented formulation for procedural sedation during cataract surgery.
Non-preserved Latanoprost Approved Thea Pharma Recently announced that the FDA approved Iyuzeh (latanoprost ophthalmic solution) 0.005% for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. The company says Iyuzeh is the only clinically-proven, non-preserved formulation of latanoprost available in the United States.
Harrow to Acquire Products from Novartis Harrow entered into an agreement to acquire the U.S. commercial rights to the following five FDA-approved products from Novartis: • Ilevro; • Nevanac; • Vigamox; • Maxidex; and • Triesence. Harrow will make a payment of $130 million at closing, with up to an additional $45 million payable upon the commercial availability of Triesence, which is expected in the second half of 2023. |
Systemic Drugs and Cataract
Speeding up the development of cataracts is just one well-known ocular complication of a number of systemic drugs, and a new study published in AJO aimed to identify the biggest culprits. The retrospective, cross-sectional design included people 40 years and older, and data from the 1999-2008 National Health and Nutrition Examination Survey was collected for analysis.
Out of the total 14,931 participants included in the analysis, 9.6 percent displayed a prevalence of surgically treated cataract (2,010 people). The researchers identified 20 different drug categories with significant association to surgically treated cataract, with eight of those 20 remaining significantly associated after adjustment for comorbidity instance.
Highest in association were the drug categories of tricyclic antidepressants, insulin and group III antiarrhythmic agents. The other categories included SSRI antidepressants, calcium channel blocking agents and loop diuretics. Providing some protection against risk of surgical cataract intervention was the use of sex hormone combinations in women. For all eight of the drug categories, dose-response relationships were present.
The authors of the study highlight that “our comprehensive evaluation provides new knowledge on the complex relationships between systemic medications and surgically treated cataract,” and elaborate by providing potential explanations for the observed associations.
As for antidepressants, while previous research shows mixed results in their effect on developing cataract, other mechanisms might be at play in conferring increased cataract risk in those taking this type of drug. That includes indirect effects of high intraocular pressure or glaucoma related to antidepressants, photosensitivity caused by them or the cataractogenic potential of serotonin.
With antidiabetic medications, mainly insulin, one suggested mechanism may be an increased photosensitivity of the lens as a result, thus leading to accelerated formation of cataract. Additionally, insulin use may cause proteins to unfold in response and subsequently epithelial cell death.
Related to diabetes, hyperglycemia is proposed to have a pathogenic role in formation and progression of cataract through the aldose reductase pathway causing hyperosmotic conditions, increased oxidative stress and inflammation and advanced lens protein glycation.
The protective effect seen in sex hormone combinations against needing surgery for cataract has its own potential explanations. One is that estrogen receptors directly interact with lens epithelial cells. Other possible options include antioxidant properties shown in sex hormones preventing formation and worsening, countering damaging effects induced by transforming growth factor ß or simply maintaining the cell membrane’s normal functioning.
Following these documented associations of systemic drugs, the authors of the study believe that their findings “could provide valuable insights into the biological mechanisms underlying the formation and progression of cataract and facilitate the future development of more effective prevention and treatment methods for cataract.
The Cataract/Strabismus Connection
About 10 percent of patients who have pediatric cataract extraction will need strabismus surgery within five years, according to a paper recently published in Ophthalmology Science that used claims data to evaluate associations and risk factors.
The researchers retrospectively analyzed claims from two insurance databases of patients ≤18 years old who underwent cataract surgery and had no history of strabismus. They found that 4.7 percent (271/5,822) of children included in the study had strabismus surgery, with a 9.6 percent cumulative incidence of strabismus surgery within five years.
Undergoing strabismus surgery was significantly associated with the following:
• younger age at the time of cataract surgery;
• female sex;
• history of persistent fetal vasculature;
• history of nystagmus;
• pre-existing strabismus diagnosis; and
• less risk of IOL placement
Though the estimated cumulative incidence for strabismus surgery after cataract surgery was lower than estimates previously described, the researchers noted that their numbers were comparable when the data was stratified by age and pre-existing strabismus diagnosis. They concluded in their paper that “future efforts toward screening would be particularly beneficial in these patients.”
Correction In January’s Medicare Q & A column, the codes for canaloplasty were incorrect. The article states: 66714- Transluminal dilation of aqueous outflow 66174- with retention of device or stent The correct codes are as follows: 66174-Transluminal dilation of aqueous outflow canal (e.g., canaloplasty); without retention of device or stent (Do not report 66174 in conjunction with 65820) -66175-with retention of device or stent. |