| ||||||||
Diagnosis, Workup and Treatment
|
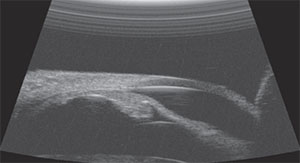
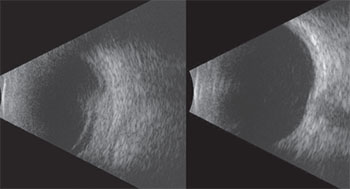
During exam under anesthesia ultrasound biomicroscopy of the anterior segment was performed, demonstrating diffuse thickening of the iris in the inferotemporal sector with no abnormality of the adjacent ciliary body (See Figure 4). The lesion measured 11 mm in base and 2 mm in thickness.
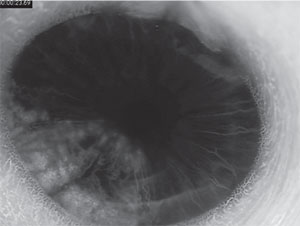
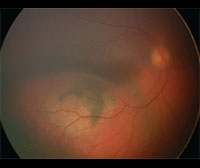
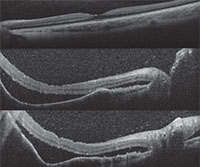
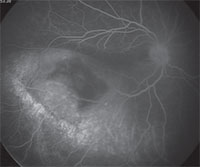
Anterior segment fluorescein angiography revealed a rapidly filling vascular lesion with no leakage of dye from the vessels (See Figure 5). Ancillary testing to this point proved revealing but non-diagnostic, and the next step in evaluating the lesion was felt to be fine needle aspiration biopsy. Prior to proceeding with biopsy, a dilated examination of the fundus was performed. The left fundus was found to be unremarkable; however the right fundus showed a diffuse, orange-red choroidal lesion involving the entire fundus posterior to the equator with greatest thickness in the inferior macula (See Figure 6) associated with an overlying serous retinal detachment; this was confirmed on optical coherence tomography (See Figure 7). Fluorescein angiography of the lesion revealed rapid filling with no leakage (See Figure 8). B-scan ultrasonography revealed an acoustically solid, echogenic mass, measuring 6.5 mm in thickness. A-scan ultrasonography of the lesion revealed high internal reflectivity characteristic of choroidal hemangioma.
Based on these findings, a diagnosis was made of diffuse choroidal hemangioma with sector hemangioma of the iris. The diagnosis was made clinically and biopsy of the lesion was not necessary.
The child was initially treated with oral propranolol (20 mg p.o. t.i.d. or 2 mg/kg/day) to assist in reduction of the choroidal hemangioma and resolution of the subretinal fluid. Care was coordinated with his pediatrician to monitor for cardiovascular complications such as bradycardia and hypotension. The choroidal tumor showed little response to oral propranolol therapy and after five months, tumor thickness was 6.1 mm from an initial thickness of 6.5 mm. Given the poor response to oral propranolol, the decision was made to treat the tumor with plaque radiotherapy. An Iodine-125 plaque was applied to the sclera overlying the tumor with an apex dose of 35 Gray centered over the thickest portion of the lesion. The plaque was left in place for four days and a single injection of intravitreal Avastin (1.25/0.05cc) was administered at the time of plaque removal. The tumor showed excellent response to this regimen, and at six weeks post- plaque choroidal tumor thickness was 3.3 mm, from an initial thickness of 6.5 mm. Further regression of the tumor was noted and at last follow-up tumor thickness was 2.6 mm (See Figure 9). There was complete regression of subretinal fluid and no recurrence of tumor after more than two years of follow-up. Despite regression of the tumor and resolution of chronic serous retinal detachment, visual acuity in the right eye remained poor at 20/400 OD. Polycarbonate lenses were recommended for protection of the better-seeing eye.
|
Discussion
|
While asymptomatic patients may be observed, patients with decreased vision secondary to subretinal fluid often benefit from intervention. Treatment options for DCH include oral propranolol, photodynamic therapy and radiation therapy (case reports of external beam radiotherapy, proton beam radiotherapy, plaque brachytherapy1 and stereotactic radiotherapy have been described).11 Oral propranolol was incidentally found to induce regression in infantile hemangiomas of the skin and, although ineffective in our patient, case reports exist documenting the utility of oral proranolol in the treatment of diffuse choroidal hemangioma.2,10 A single report exists in the literature regarding treatment of diffuse choroidal hemangioma with isolated anti-VEGF therapy.11
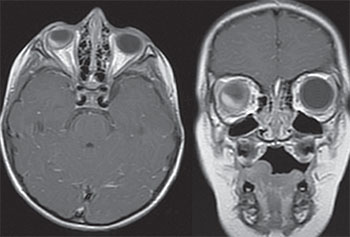
While circumscribed choroidal hemangioma is often isolated, diffuse choroidal hemangioma is commonly found in association with Sturge-Weber syndrome, a rare, congenital, nonhereditary neuro-oculo-cutaneous syndrome associated with facial angioma (nevus flammeus or port wine birthmark), leptomeningeal angioma and vascular malformation of the eye. Our patient did not have the classic port wine birthmark and MRI of the brain revealed an absence of leptomeningeal angioma (See Figure 10). While Sturge-Weber syndrome with isolated cutaneous or leptomeningeal findings has been described, to our knowledge isolated ocular findings are extraordinarily rare.
Although diffuse hemangioma of the choroid is a common ocular manifestation of Sturge-Weber syndrome, hemangioma of the iris is exceedingly rare.3,6,12 A single other report of iris involvement has been published.5
Sturge-Weber syndrome is speculated to be due to a somatic mosaic mutation affecting vascular development in geographically related tissues with the extent of involvement determined by the developmental stage at which the somatic mutation occurs. In 2013, Matthew Shirley, PhD, and colleagues described a single nucleotide mutation in GNAQ present in samples of affected tissue of patients with Sturge-Weber syndrome and non-syndromic port wine birthmarks.7 While our patient’s clinical findings are not classic for Sturge-Weber syndrome, they likely represent a spectrum of disease that occurs when the presumed somatic mutation occurs at a later stage in development affecting the eye only.
This case highlights the importance of dilated fundus examination in pediatric patients and suggests that clinicians should have a low threshold for examination under anesthesia for children with poor vision and a difficult exam. REVIEW
1. Arepalli S, Shields CL, Kaliki S, Komarnicky L, Shields JA. Diffuse choroidal hemangioma management with plaque radiotherapy. Ophthalmology 2013;120:2358-9.
2. Arevalo JF, Arias JD, Serrano MA. Oral propranolol for exudative retinal detachment in diffuse choroidal hemangioma. Arch Ophthalmol 2011;129:1373-1375.
3. Ferry AP. Hemangiomas of the iris and ciliary body. Do they exist? A search for a histologically proved case. Int Ophthalmol Clin 1972;12:177-94
4. Krema H1, Yousef YA, Durairaj P, Santiago R. Failure of systemic propranolol therapy for choroidal hemangioma of Sturge-Weber syndrome: A report of 2 cases. JAMA Ophthalmol 2013 May;131(5):681-3.
5. Shields CL, Atalay HT, Levin AV, Wuthisiri W, Lally SE, Shields JA. Sector iris hemangioma in association with diffuse choroidal hemangioma. J AAPOS 2015 Feb;19(1):83-6. doi: 10.1016/j.jaapos.2014.09.012.
6. Shields CL, Kancherla S, Patel J, et al. Clinical survey of 3680 iris tumors based on patient age at presentation. Ophthalmology 2012;119:407-14.
7. Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Eng J Med 2013;368:1971-9.
8. Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanism of action. Br J Dermatol 2010; 163(2):269-274.
9. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus 1992;29:349-56.
10. Thapa, R, Shields CL. Oral propranolol therapy for management of exudative retinal detachment from diffuse choroidal hemangioma in Sturge-Weber syndrome. Eur J Ophthalmol 2013 Jun 2;23(6):922-924.
11. Tsipursky MS, Golchet PR, Jampol LM. Photodynamic therapy of choroidal hemangioma in sturge-weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv Ophthalmol 2011;56(1):68-85.
12. Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Opthalmol 1976;20:415-31.