Presentation
A 52-year-old male with several months of decreasing vision in the right eye is referred to the Wills Eye cornea clinic. He was struck in the right eye by a tree branch while hiking five months prior, for which he was treated with antibiotic drops. After the initial healing, he noted a progressive decline in his vision. He denied any pain, redness, discharge or other ocular symptoms.
History
The patient had an ocular history notable for LASIK in both eyes more than 20 years ago. He also underwent a basal cell carcinoma excision of the left lower eyelid two weeks prior to presentation. Past medical history was only notable for hyperlipidemia, managed with rosuvastatin. Family history was non-contributory. The patient never smoked or used alcohol. Review of systems was unremarkable.
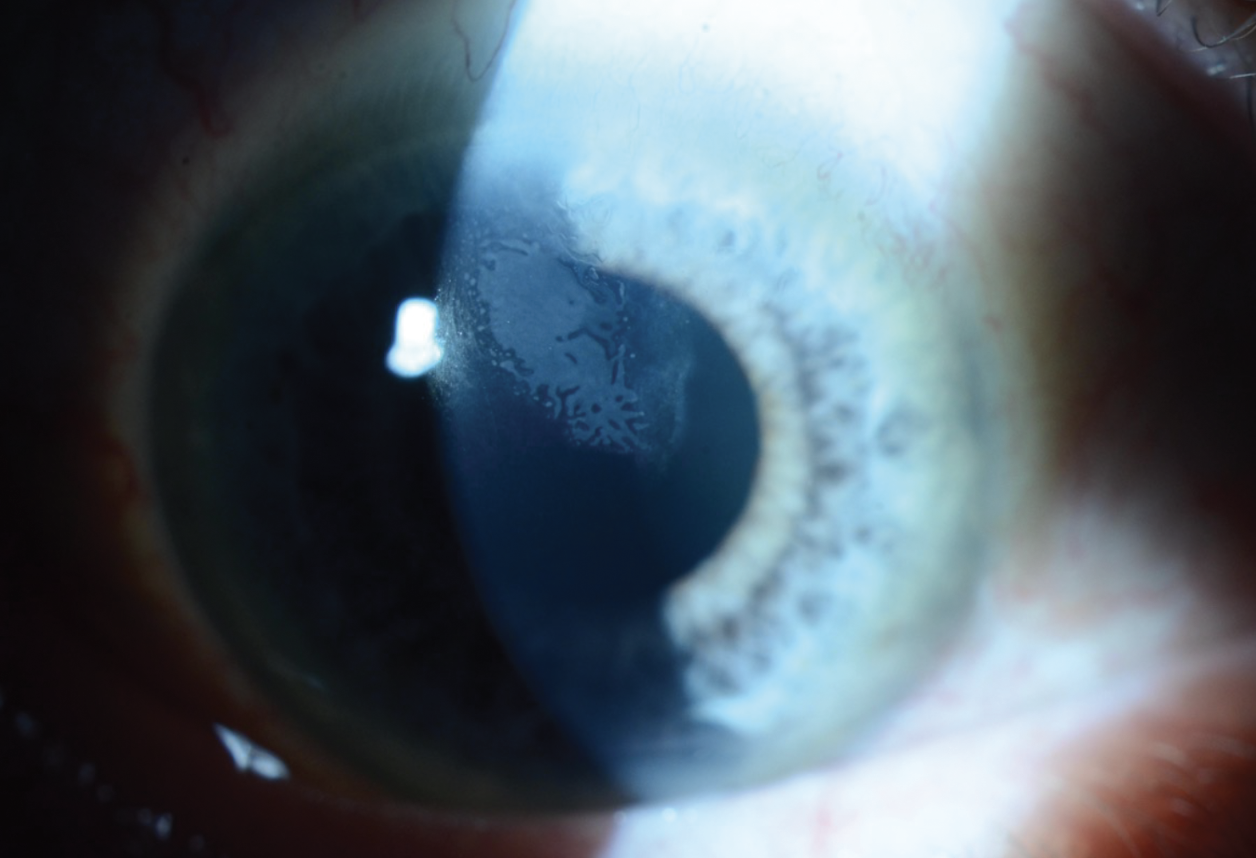 |
| Figure 1. Slit lamp exam photograph of the patient’s right eye showing a superior opacity extending from the superior LASIK flap edge. |
Examination
At presentation, uncorrected visual acuity was 20/50 in the right eye and 20/60 in the left. His pupils were round and reactive in both eyes without an afferent pupillary defect in either eye. Intraocular pressures were 12 mmHg and 14 mmHg in the right and left eyes, respectively. Extraocular motility and confrontation visual fields were full bilaterally.
Slit lamp examination of the right eye revealed a 4 mm (width) by 3.7 mm (height) creamy-white opacity extending from the superior flap edge (Figure 1). High magnification slit-beam view revealed the opacity was in the LASIK flap interface. There was no cleft at the LASIK flap edge. The remainder of the anterior exam of the right eye was unremarkable. Anterior segment examination of the left eye was notable for a well-healing lower lid excision with intact sutures in place, and a nasal-hinge LASIK flap well-positioned and without opacities. Topography of the right eye showed irregular astigmatism in the area of the corneal opacity (Figure 2).
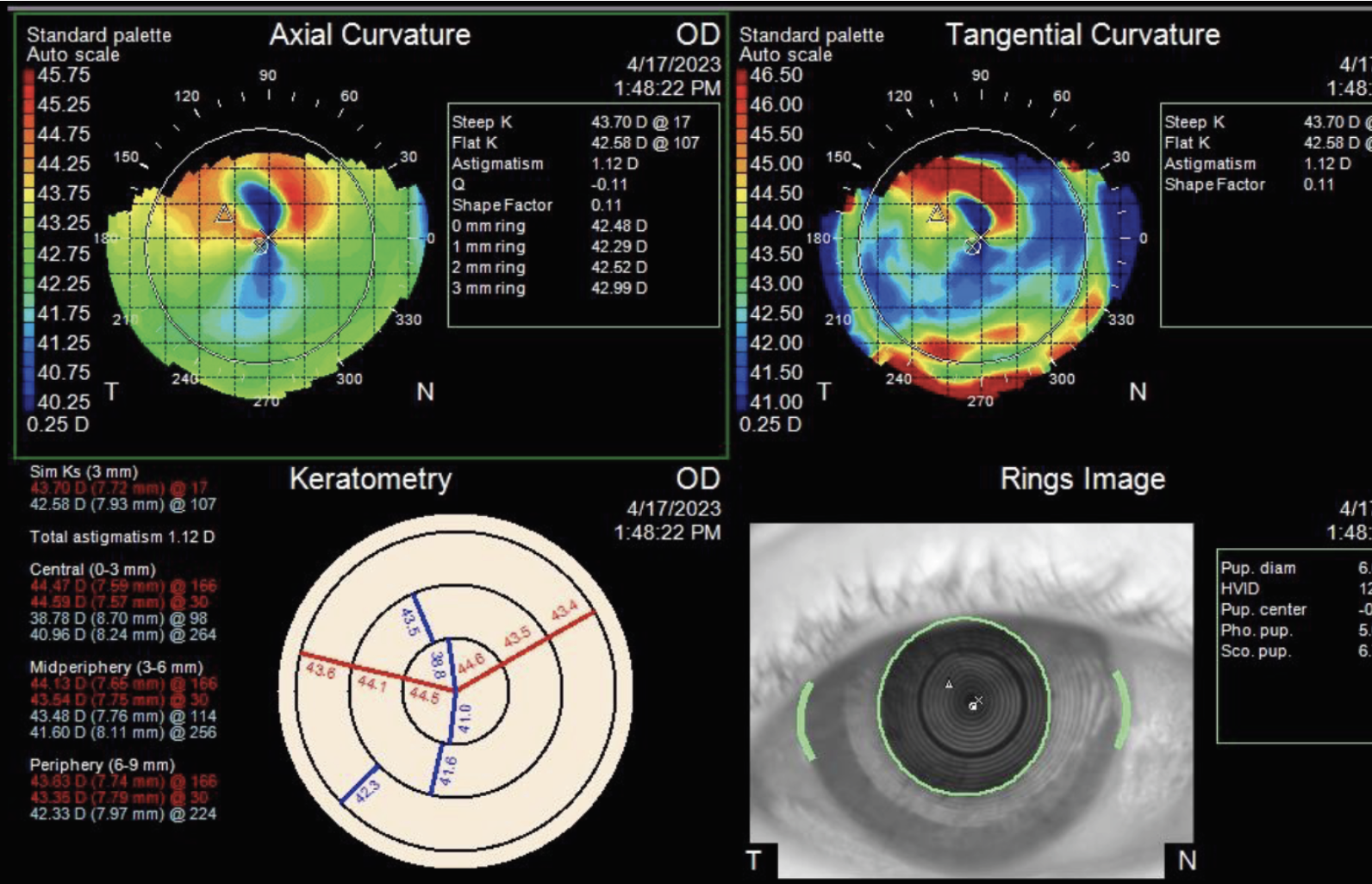 |
| Figure 2. Topographic report of the right eye showing irregular astigmatism in the area of the corneal opacity. |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
The differential diagnosis includes epithelial basement membrane dystrophy, epithelial ingrowth in the LASIK flap interface, diffuse lamellar keratitis and infectious keratitis. Given the patient’s clinical history, unilaterality, slit lamp examination and otherwise quiet anterior segment exam, post-LASIK epithelial ingrowth (PLEI) secondary to flap-dislocation from recent trauma was the diagnosis. Given the extent of the epithelial ingrowth and progressive decreasing vision, treatment was recommended.
The patient underwent flap lift in the right eye with mechanical debridement of the ingrowth, followed by suturing of the flap edge. Histopathology of a surgical specimen demonstrated partially degenerated corneal epithelium compatible with epithelial ingrowth (Figure 3). On postoperative day one, the patient’s uncorrected visual acuity was 20/60 in the right eye. He started on besifloxacin four times daily and prednisolone acetate 1% four times daily for one week. At the patient’s postoperative week one visit, the besifloxacin was stopped, and the prednisolone acetate 1% was tapered over the course of two weeks. His anterior segment exam remained stable with a well-positioned LASIK flap with no epithelial interface or stromal infiltrate and seven intact sutures in place (Figure 4). The sutures were removed over two visits at postoperative months two and three. At postoperative month four, the patient’s uncorrected visual acuity in the right eye was 20/30 -1. Anterior segment exam showed a well-positioned LASIK flap without epithelial cells centrally but with a few cells at the superior flap edge extending < 0.3 mm. At postoperative month six, the patient’s uncorrected visual acuity was 20/30 +3 and his anterior segment exam was stable without changes.
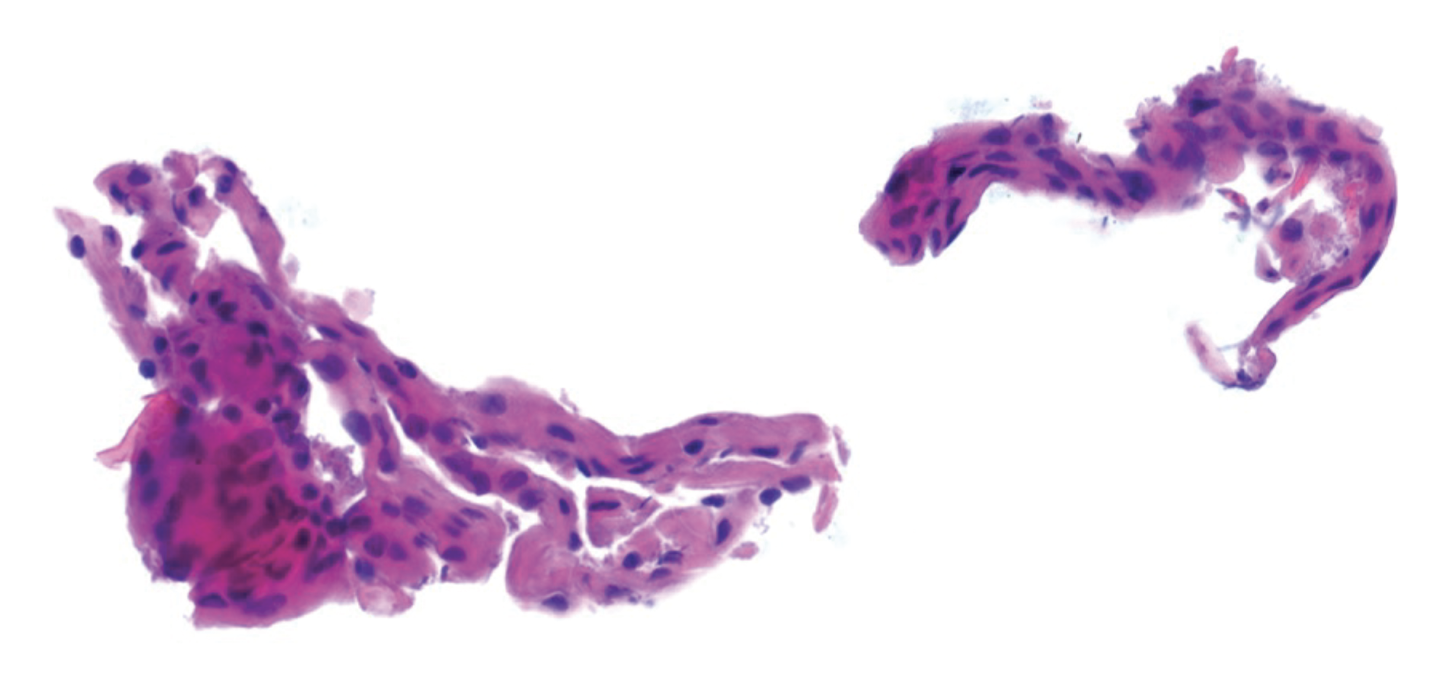 |
| Figure 3. Histopathology of a surgical specimen from mechanical debridement of the lifted LASIK flap showing corneal epithelium compatible with epithelial ingrowth. |
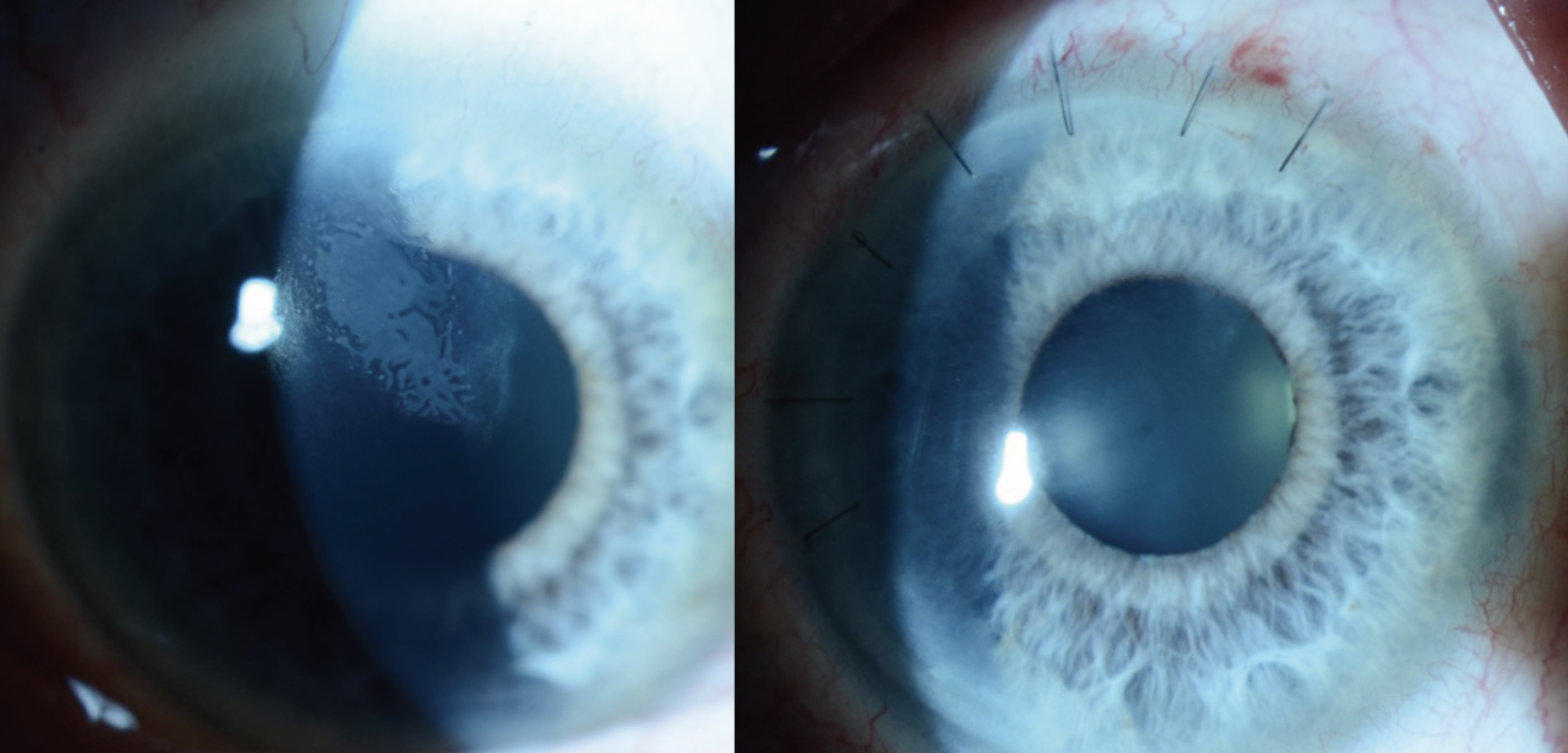 |
| Figure 4. Slit lamp exam photographs of the patient’s eye before (left) and one week after (right) mechanical debridement of epithelial ingrowth, showing resolution of the interface opacities. |
Discussion
Post-LASIK epithelial ingrowth is a rare complication following LASIK surgery, with an incidence measuring between 0 to 3.9 percent.1 Though most cases are generally detected within two months of surgery, rare cases of late-onset PLEI have been reported following traumatic injuries.2,3 Risk factors for the development of PLEI can be broadly divided into modifiable and non-modifiable factors. Modifiable risk factors are largely related to operative technique and include surgical instrumentation, method of flap manipulation and conformation of the flap edge. Non-modifiable risk factors are less well-studied but may include patient factors such as age and diabetes status, type of refractive correction and flap lifts for retreatment.1,4,5
The management of PLEI can be guided using the Probst/Machat grading system, which considers the appearance and location of the epithelial ingrowth (Table 1). Indications for treatment include decreased vision, often from irregular astigmatism, and damage to the LASIK flap. Damage can result from dense ingrowth impeding nutrients from getting to the flap causing injury ranging from punctate epitheliopathy to epithelial defect to flap melting. Grade 1 cases aren’t visually significant, rarely progress and can be managed with observation. Grade 2 cases may slowly progress and eventually impact vision, warranting non-urgent treatment within weeks or months. Grades 3 and 4 cases often significantly impact vision and can rapidly progress, requiring urgent treatment.
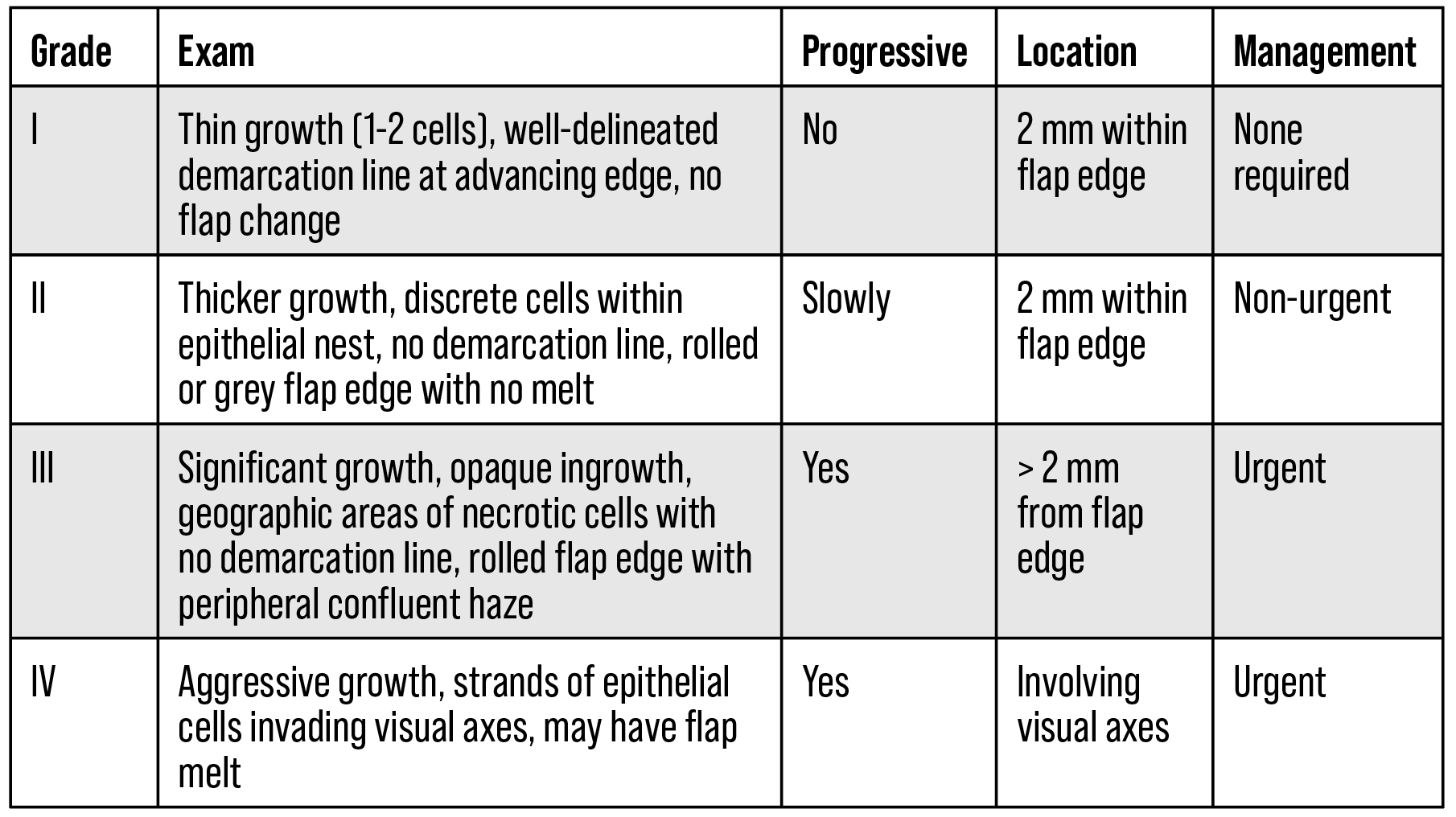 |
| Table 1. Probst/Machat grading system for PLEI (adapted from Ting et. al., modified from Neff and Probst).1,6 |
When treatment is indicated, the most common and well-studied intervention is mechanical debridement. In studies with greater than 10 treated eyes, surgical debridement achieved uncorrected distance VA ≥ 20/60 in 74 to 80 percent of cases and ≥ 20/25 in 45 to 53 percent of cases, and corrected distance VA ≥ 20/60 in 78 to 91 percent of cases and VA ≥ 20/25 in 78 to 85 percent of cases.1 During debridement, care is taken to scrape both the exposed stroma and the posterior side of the flap free of epithelial cells. Adjuvant ethanol, isopropyl alcohol, mitomycin C and excimer laser phototherapeutic keratectomy have all been described in various case studies, without statistically significant differences in outcomes.2,7-9 At Wills, mechanical debridement without adjuvant therapy with or without suturing of the flap edge is the standard practice. Some cases of PLEI can also be treated with non-invasive neodymium:yttrium aluminum garnet (Nd:YAG) laser, though outcomes are less consistent compared to mechanical debridement.10,11
In conclusion, PLEI is a rare but potentially visually significant complication following LASIK surgery that can present years after surgery, often in the setting of flap-lift LASIK enhancements and traumatic LASIK flap displacement. We describe a patient with months of gradual vision loss after a traumatic injury to his eye 20 years after LASIK surgery. Mechanical debridement with or without flap edge suturing remains the standard of care at Wills and often results in excellent outcomes.
1. Ting DSJ, Srinivasan S, Danjoux J-P. Epithelial ingrowth following laser in situ keratomileusis (LASIK): Prevalence, risk factors, management and visual outcomes. BMJ Open Ophthalmology 2018;3:e000133.
2. Rapuano CJ. Management of epithelial ingrowth after laser in situ keratomileusis on a tertiary care cornea service. Cornea 2010;29:307–13.
3. Holt DG, Sikder S, Mifflin MD. Surgical management of traumatic LASIK flap dislocation with macrostriae and epithelial ingrowth 14 years postoperatively. J Cataract Refract Surg 2012;38:357–61.
4. Asano-Kato N, Toda I, Hori-Komai Y, et al. Epithelial ingrowth after laser in situ keratomileusis: Clinical features and possible mechanisms. Am J Ophthalmol 2002;134:801–7.
5. Jabbur NS, Chicani CF, Kuo IC, et al. Risk factors in interface epithelialization after laser in situ keratomileusis. J Refract Surg 2004;20:343–8.
6. Neff KD, Probst LE. In: LASIK complications. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Surgery of the cornea and conjunctiva, 3rd ed. St. Louis: Mosby, 2011:1861.
7. Güell JL, Verdaguer P, Mateu-Figueras G, et al. Epithelial ingrowth after LASIK: Visual and refractive results after cleaning the interface and suturing the lenticule. Cornea 2014;33:1046–50.
8. Lahners WJ, Hardten DR, Lindstrom RL. Alcohol and mechanical scraping for epithelial ingrowth following laser in situ keratomileusis. J Refract Surg 2005;21:148–51.
9. Yeh DL, Bushley DM, Kim T. Treatment of traumatic LASIK flap dislocation and epithelial ingrowth with fibrin glue. Am J Ophthalmol 2006;141:960–2.
10. Ayala MJ, Alió JL, Mulet ME, et al. Treatment of laser in situ keratomileusis interface epithelial ingrowth with neodymium:yytrium-aluminum-garnet laser. Am J Ophthalmol 2008;145:630–4.
11. Kim JM, Goel M, Pathak A. Epithelial ingrowth—Nd:YAG laser approach. Clin Exp Ophthalmol 2014;42:389–90.