Diabetic retinopathy is the leading cause of blindness in patients aged 20-74 years in the United States.1 Macular edema affects approximately 29 percent of diabetic patients with disease duration of 20 years or more and is the main reason of reduced vision in this population. In the Early Treatment Diabetic Retinopathy Study, the three-year risk of moderate visual loss (a doubling of the initial visual angle or a decrease of three lines or more on a logarithmic visual acuity chart) for diabetic patients with clinically significant macular edema was 30 percent.
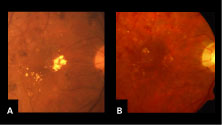 | |
| Figure 1. A: High-magnification clinical photograph at baseline of the same patient demonstrated the presence of hard exudates in the macula. B: High-magnification clinical photograph six months after IVTA of the same eye revealed marked resolution in the presence of hard exudates in the macula. | |
This article reviews the rationale behind using intravitreal trimacinolone to treat diabetic macular edema and the research results that have been reported.
Types of DME
Two different subtypes of diabetic macular edema are recognized. Focal macular edema is characterized by focal leakage from microaneurysms and is often associated with intraretinal lipid deposition in a cyrcinate pattern. Diffuse macular edema is characterized by diffuse leakage from the retinal capillaries and formation of cystoid spaces. In eyes with diffuse macular edema, laser treatment cannot be focused on localized leaking microaneurysms, since there is diffuse leakage from the capillary bed and thickening of the entire macula. In the ETDRS, grid laser treatment was applied to areas of diffuse macular edema. Previous studies have shown, however, that eyes with diffuse macular edema carry a particularly poor prognosis despite laser photocoagulation.3
Optical coherence tomography offers a method of high-resolution cross-sectional imaging of the retina utilizing light to detect relative changes in reflection at optical interfaces. OCT has proven to be a useful technique for quantifying macular thickness in patients with diabetic macular edema.4 Further, central macular thickness measured by OCT has been correlated with visual function.5
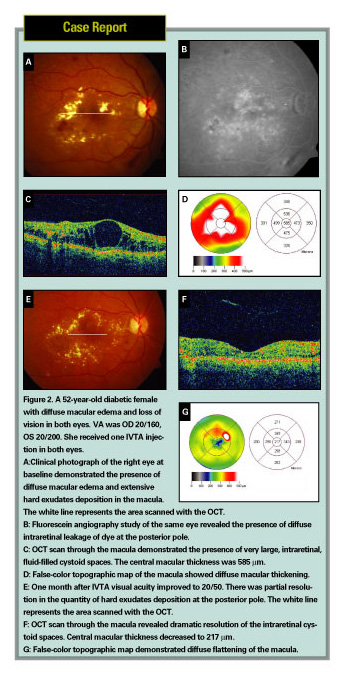 |
Rationale for Corticosteroids
Robert Machemer, MD, first advocated the use of intravitreal corticosteroid for the treatment of proliferative vitreoretinopathy.6 Brooks McCuen, MD, and colleagues demonstrated the lack of ocular toxicity of intravitreal triamcinolone acetonide in an experimental rabbit model.7 Their results agreed with clinical observation of cases in which corticosteroids were accidentally injected into the eye, and no major toxic reactions were detected.8 For the past several decades since that time, intravitreal corticosteroids have been used in limited trials in patients without evidence of ocular toxicity.
Triamcinolone acetonide is a corticosteroid suspension that has been used in periocular injections for the treatment of cystoid macular edema secondary to uveitis and as a result of intraocular surgery. Intravitreal triamcinolone acetonide has also been used for the treatment of uveitic cystoid macular edema,9 exudative age-related macular degeneration,10 neovascular glaucoma,11 proliferative diabetic retinopathy,12 hypotony,13 proliferative vitreoretinopathy,14 macular edema secondary to retinal vascular occlusive disease,15 pseudophakic cystoid macular edema, and cystoid macular edema in retinitis pigmentosa.16 IVTA has also been reported to have favorable results in the treatment of diffuse diabetic macular edema.17,18
The exact mechanism of action of corticosteroids in the treatment of macular edema is unknown. However, the rationale behind their use lies in their ability to inhibit the arachidonic acid pathway, of which prostaglandin is a product. Corticosteroids may also downregulate the production of vascular endothelial growth factor.
Finally, triamcinolone acetonide has been shown experimentally to reduce the breakdown of the blood-retinal barrier.19 It is accepted that in diabetic macular edema there is a breakdown of the blood-retina barrier, and that prostaglandins and VEGF may play a role in this process. There is indeed, a theoretic rationale for the use of corticosteroids in the treatment of diabetic macular edema.
Triamcinolone Studies
Adam Martidis, MD, and his coworkers recently reported on the use of IVTA at the dose of 4 mg in 0.1 mL for the treatment of refractory diabetic macular edema.18 Sixteen eyes were enrolled in their study, and 14 of the 16 eyes completed one month follow-up; eight of the 16 eyes (50 percent) completed six months follow-up. The investigators found a mean improvement in visual acuity of 2.4, 2.4, and 1.3 Snellen lines at the one-, three- and six-month follow-up intervals, respectively. The central macular thickness as measured by OCT decreased by 55 percent, 57.5 percent and 38 percent respectively over these same intervals from an initial pretreatment mean ± SD of 540.3 mm ± 96.3 mm. Intraocular pressure exceeded 21 mmHg in five, three and one eye respectively during these intervals. One eye exhibited cataract progression at six months. No other complications were noted over a mean follow-up of 6.2 months. Reinjection was performed after six months in three of eight eyes (37 percent), because of recurrence of diabetic macular edema.
Jost Jonas, MD, and colleagues reported similar results with the use of IVTA at the dose of 25 mg in 0.2 mL in diabetic patients with diffuse macular edema.17 They enrolled 26 eyes of 20 patients. Mean ± SD follow-up was 6.64 ± 6.10 months. Mean ± SD visual acuity improved from 0.12 ± 0.08 at baseline to 0.19 ± 0.14 during follow-up. One patient received a second intravitreal injection of 25 mg of triamcinolone acetonide. For the 22 patients for whom fluorescein angiograms were available during the preinjection and postinjection periods, a significant decrease in the fluorescein leakage was noted after IVTA. During the study period intraocular pressure raised above 21 mmHg in nine (34.6 percent) of the 26 study eyes.
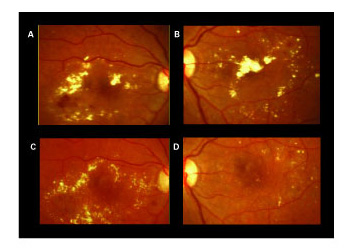 |
| Figure 3. A and B: High-magnification color photographs (top) show hard exudates in the macula of both eyes. C and D: One month (OD), and two months (OS) after IVTA demonstrated partial resolution in the hard exudates deposition. |
IVTA for DME
In our personal clinical experience, we have been using IVTA for the treatment of refractory diabetic macular edema. The intravitreal injection is performed under topical anesthesia with proparacaine eye drops. A lid speculum is used to keep the eyelashes away from the conjunctiva. Povidone iodine 5% eye drops and Ocuflox eye drops are instilled in the conjunctiva every five minutes for three times. The injection of triamcinolone acetonide 4 mg in 0.1 mL is performed through a 30-ga. needle in the inferotemporal pars plana 3.5 mm posterior to the limbus.
After the injection, indirect ophthalmoscopy fundus examination is used to evaluate the perfusion of the central retinal artery and the intravitreal location of the triamcinolone. We have noted resolution of the macular edema and improvement in vision after IVTA.
These data suggest that IVTA is effective in improving visual acuity and reducing macular thickness measured by OCT. Such a positive effect reaches its maximum one to three months after the IVTA treatment. This is followed by a decline in vision and increase in macular thickness over the following months.
Repeated IVTA treatment is effective in determining new improvement in visual acuity. These data are consistent with the work of Paul Beer, MD, and colleagues, who showed that in human eyes after intravitreal injection, measurable concentrations of triamcinolone would be expected to last for approximately three months (93 ± 28 days) in a non-vitrectomized eye.22
IVTA is a promising therapy for patients with diabetic macular edema refractory to laser treatment. IVTA is effective in improving vision, reducing macular thickness and inducing reabsorption of hard exudates. Endophthalmitis is the most serious complication of IVTA, but fortunately did not occur in this study. Further investigation is warranted to assess the efficacy and safety of IVTA for the treatment of refractory diabetic macular edema.
Dr. Ciardella practices at the Denver Health Medical Center, the Rocky Mountain Lyon Eye Institute, Aurora, Colo., and the University of Colorado Health Sciences Center, Denver. Contact him at Denver Health Medical Center, 777 Bannock St., Mail Code 0156, Denver, Colo. 80204. Phone: (303) 436-3448; fax: (303) 436-3181; e-mail: Antonio.ciardella@dhha.org. Dr. Chang practices at Columbia University, New York. Neither author has any financial interest in the subject matter.
1. Chew EY, Ferris III FL. Nonproliferative diabetic retinopathy. In: Ryan SJ, ed. Retina. St. Louis: Mosby Inc., 2001:1295-1308.
2. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol 1985;103:1796-1806.
3. Bresnick GH. Diabetic maculopathy: a critical review highlighting diffuse macular edema. Ophthalmology 1983;90:1301-1317.
4. Hee MR, Puliafito CA, Duker JS, et al. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology 1998;105:360-370.
5, Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S. Morphological and functional analysis of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefe's Arch Clin Exp Ophthalmol 2001; 239:96-101.
6. Machemer R, Sugita G, Tano Y. Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc 1979;77:171-180.
7. McCuen BW II, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol 1981;91:785-788.
8. Giles CL. Bulbar perforation during periocular injection of corticosteroids. Am J Ophthalmol 1974;77:438441.
9. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology 2001;108:765-772.
10. Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2003;110:1517-1525.
11. Jonas JB, Hayler JK, et al. Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. J Glaucoma 2001;10:284-287.
12. Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol 2001;131:468-471.
13. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as treatment of pre-phthisical ocular hypotony. Graefe's Arch Clin Exp Ophthalmol 2001;239:464-465.
14. Tano Y, Sugita G, Abrams G, Machemer R. Inhibition of intraocular proliferation with intravitreal corticosteroid. Am J Ophthalmol 1980;89:131-136.
15. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular edema due to central retinal vein occlusion. Br J Ophthalmol 2002;86:247-248.
16. Saraiva VS, Sallum JM, Farah ME. Treatment of cystoid macular edema related to retinitis pigmentosa with intravitreal triamcinolone acetonide. Ophthalmic Surg Lasers Imaging 2003;34:398-400.
17. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 2003;121:57-61.
18. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002;109:920-927.
19. Wilson CA, Berkowitz BA, Sato Y, et al. Treatment with intravitreal steroids reduces blood-retina barrier breakdown due to laser photocoagulation. Arch Ophthalmol 1992;110:155-159.
20. Roth DB, Chieh J, Spirn MJ, Green SN, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol 2003;121: 1279-1282.
21. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 2003;136:791-796.
22. Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003;110:681-686.



