The burdens glaucoma imposes on patients, families and ophthalmologists continue to increase: The global incidence of open-angle glaucoma is anticipated to reach 65.5 million cases by 2020.1 Speaking at the recent 2018 American Society of Cataract and Refractive Surgery summit in Washington, D.C., Stanford University’s Kuldev Singh, MD, noted that despite variations in the natural history of the disease from patient to patient, individuals are living longer with glaucoma, uniformly increasing the risk of progression to blindness. Lowering a patient’s mean IOP is currently the first defense against progression, but a user-friendly, round-the-clock IOP-monitoring system remains just out of reach. Here, experts discuss the state of 24-hour IOP monitoring devices meant for use outside the clinic.
“Considerations include portability, costs, ease of use and accuracy,” said Dr. Singh at the ASCRS summit. Two devices with relatively recent FDA approval facilitate home IOP monitoring, each with its own capabilities and limitations. Another internal IOP monitor, CE marked in Europe, may prove to advance the quest for user-friendly monitoring further still. Cost may remain a barrier to out-of-clinic IOP monitoring in medically underserved populations, however, and so a computer scientist has devised a prototype in the hopes of making basic IOP monitoring accessible globally.
The Icare Home
The Icare Home (Icare USA; Raleigh, North Carolina), which gained FDA approval in March of 2017, is a rebound tonometer that doesn’t require the user to instill a topical anesthetic. The patient depresses a button, and a small probe with a soft, disposable cover quickly touches the ocular surface before retracting back into the tonometer. Automatic OS/OD recognition and red and green lights guide proper alignment of the device over the eye, and patients can select the device’s sequencing to take one reading at a time or a cluster of six in rapid succession. The Icare Home stores data, which health care providers can retrieve using proprietary software.
A 2017 study2 compared Icare Home measurements done by 130 glaucoma patients and suspects to measurements done by an ophthalmologist using the device and Goldmann Applanation Tonometry (GAT) measurements done by an ophthalmologist. Ninety-eight percent (128/130) of patients
 |
were able to use the Icare properly. Mean IOP ranges were 7 to 20 mmHg with GAT; 6 to 24 mmHg with Icare measurements done by the patient, and 6 to 25 mmHg with Icare measurements done by the ophthalmologist. The mean difference between HOMEp (patient) and HOMEo (ophthalmologist) was 0.21 mmHg (p=0.068; paired t-test). The mean difference between the HOMEp and GAT measurements was 0.70 mmHg (p<0.001; paired t-test), and between the HOMEo and GAT measurements it was 1.00 mmHg (p<0.001; paired t-test).2 The investigators found that the Icare Home tonometer demonstrated a tendency to capture higher IOP values than GAT overall, but deemed it feasible for self-monitoring IOP. Another study suggested that patients could use the Icare Home comparably to a specialist and that they found it acceptable to use.3
“That [latter] study assessed patient acceptability of using self-tonometry,” says Kaweh Mansouri, MD, MPH, who practices at the Glaucoma Centre, Montchoisi Clinic, Lausanne, Switzerland, and serves as an adjunct associate professor at University Colorado-Denver. “They found that most patients were happy to use this device themselves, and that the measurements they obtained were similar to what the physician would measure using the same device.”
Dr. Mansouri acknowledges that the Icare is limited by the patient’s activities and wakefulness. “The Icare measures IOP in snapshots. It’s not continuous, and it doesn’t measure IOP while the patient is asleep, or during different activities,” he notes.
Steven L. Mansberger, MD, MPH, vice chair and director of the glaucoma service at Legacy Devers Eye Institute in Portland, Oregon, doesn’t think evidence of the Icare Home’s therapeutic value is compelling enough for him to recommend it to patients at this time. “The Icare device is validated in some ways, but in other ways, we don’t really understand how it corresponds with glaucoma,” he says. “It doesn’t really fully assess the risk factor of intraocular pressure, meaning that it doesn’t check it at night. It doesn’t check it when the patient is lying supine. It doesn’t allow pressure measurements during normal activities like showering or exercise or things like that. So it’s unclear how the device itself really represents this risk factor that we call ‘elevated intraocular pressure.’ If patients check their pressure three or four times a day, how much value does that add? That’s what we really haven’t been able to figure out,” he notes.
The Icare Home that’s commercially available in the United States stores data internally, so patients aren’t able to see their IOP readings. At ASCRS this spring, Dr. Singh opined that this is an important consideration if you’re going to integrate round-the clock IOP monitoring into your practice. “Make sure you have control of the data, or the patient may become overly fixated on data,” he said. “Patients fixate on IOP and not vision; and they over-fixate on IOP.”
“People can get neurotic about their pressures,” Dr. Mansberger concurs. “To paraphrase a noted glaucoma specialist, ‘Ask not what your pressure is, but ask how your optic nerve or your visual field looks.’ It could be that variability in pressure is a normal physiologic phenomenon that has nothing to do with glaucoma’s progression,” he observes, although he adds that home monitoring of IOP could encourage people to make lifestyle changes that may help control their glaucoma, just as home glucose monitoring can encourage patients with diabetes to take better care of their health.
Triggerfish CLS
The Triggerfish contact lens sensor (Sensimed AG; Lausanne, Switzerland) consists of a soft, single-use contact lens available in three base curves (8.4, 8.7, and 9 mm), measuring 14.1 mm in diameter and 585 um thick in the central zone. The lens incorporates two strain gauges, a microprocessor, and single-use adhesive that fits around the patient’s orbit and holds a receiver antenna. There’s also a disposable recorder sleeve for the microprocessor. Intended to be worn for up to 24 hours at a time, the Triggerfish lens has a telemetric sensor that takes 30 seconds of readings at five-minute intervals over each 24-hour period of wear.
Unlike the Icare Home, the
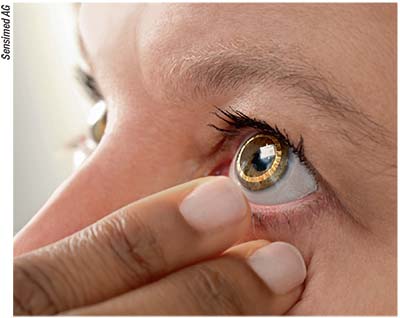 |
| The Triggerfish contact lens sensor, shown here without the receiver taped around the orbit, has tiny strain gauges and telemetry circuits embedded inside. |
Triggerfish passively records data while the patient performs waking activities or sleeps. Importantly, it’s not an IOP monitor in the strict sense of the term: The FDA approved the Triggerfish as a diurnal pattern recorder in 2017. It captures IOP fluctuations in millivolts, rather than measuring absolute IOP in millimeters of mercury.
“The Icare measures IOP in absolute values, while the Triggerfish measures different parameters, of which IOP is one,” explains Dr. Mansouri. “The Triggerfish measures strain differences. You could also call it ‘strain tonometry.’ Strain differences, which the Triggerfish measures, reflect changes in IOP, but also changes in intraocular volume; and they reflect ocular elasticity and the rigidity of the globe. All three of these are included in the Triggerfish signal. And rather than millimeters of mercury, they are relative values. So you cannot compare them one to one with conventional tonometry. This may be both a disadvantage and an advantage. The disadvantage is, of course, that you can’t obtain the standard units of tonometry. The advantage is that these other factors that the Triggerfish measures—these strain-related factors—may be a more accurate reflection of any given eye’s susceptibility to glaucomatous damage,” he says.
Dr. Mansouri cites a study4 that showed how the IOP-related measurements that the Triggerfish collected correlated with glaucoma’s progression in a group of patients with treated disease—a correlation that could help ophthalmologists identify patients in need of ongoing enhanced monitoring. “This study showed that the Triggerfish signal was related to patient progression, meaning that one session of Triggerfish monitoring could help predict which patients might be at risk for having fast glaucoma progression in the future,” he says. “The study points to the fact that it probably measures more than just IOP, and that these measurements may be a more accurate reflection of the susceptibility of an eye to suffer from glaucomatous progression in the future.”
Because patients don’t have to stop their activities to take a reading while wearing the Triggerfish, the data it collects may reveal associations between IOP spikes and certain activities and postures. Dr. Mansberger cites the work of J. Crawford Downs, PhD, and colleagues on the continuous telemetric IOP monitoring of rhesus macquaques5,6 as evidence that blinking and circadian rhythms cause IOP fluctuation, and that postural changes may have significant and persistent effects on IOP in humans. “They show that with a blink reflex, the pressure goes high; with sleep, the pressure becomes really stable. Activity creates lots of variability in the pressures. From his data, it suggests that it’s common for eye pressure to fluctuate quite widely,” he says.
Dr. Mansouri, who uses the Triggerfish for short-term round-the-clock monitoring of patients, relates how the contact lens sensor’s tracking of IOP fluctuations in the course of different activities can influence patient behavior. “One patient would practice yoga, including headstand positions,” he recalls, “and we’d measure the IOP around the clock with the Triggerfish. You could see how the values just skyrocketed when that patient assumed specific yoga positions. Based on that, it was easier to say, ‘Maybe you should refrain from those specific positions where you know your IOP will become elevated,’ ” he says.
Another study of head-down yoga poses and continuous IOP monitoring also suggests that these postures induce IOP spikes, so ophthalmologists might consider recommending against their performance in certain patients.7 Dr. Mansouri says that the Triggerfish and the Icare might both help influence behavior in patients who’ve been progressing despite normal in-clinic values. “Glaucoma is very abstract,” he says. “Patients don’t see anything. They don’t see their visual fields or atrophy getting worse. Aside from measuring IOP itself and knowing how IOP behaves, it’s very abstract. These tools can help the patient to remain adherent. The less abstract we can make the disease, the better for adherence. One way of doing so is to empower patients by allowing them to obtain IOP measurements, or by allowing them to have a look at their IOP measurements.”
Dr. Mansberger notes that the Triggerfish is not very user-friendly. “The Triggerfish is tough,” he says. “It’s not too comfortable. The patient has to wear something almost like a probe with electrodes coming out of it above their eye. It’s not very comfortable; it’s not very attractive. You can’t use it for very long because of irritation from the contact lens,” he says. Reported adverse events associated with wearing the Triggerfish contact lens sensor include corneal epithelial defects, conjunctival erythema and pain, although these resolved soon after discontinuation of use.8
The Eyemate
The Eyemate (Implandata; Hannover, Germany), CE-certified in 2017 for commercial use in the European Union, is an implantable device intended for continuous, long-term IOP monitoring. A flexible, ring-shaped sensor 12 mm in diameter is designed for sulcus implantation at the time of cataract surgery through the surgical incision. Seated in front of the intraocular lens and behind the iris, the device doesn’t impede vision and remains inconspicuous, according to the manufacturer. The other component of the Eyemate is a small handeld mesograph that telemetrically reads IOP when the patient holds it close to the implanted eye. The mesograph displays IOP measurements and stores them. When fitted with a GSM module, the Eyemate can also transfer measurements to an Internet database to share round-the-clock records with the ophthalmologist.
Dr. Mansouri currently uses the Eyemate for research purposes. He says that the underlying concept is simple. “The company took sensors used in the automobile industry in Germany to measure tire pressure. They miniaturized those for implantation in front of an IOL in the sulcus,” he explains.
“Two big studies have been conducted showing that the device was safe during surgery and over the long term,” he continues. “The company has about five years of data showing that the device stays in the eye, is well tolerated, and is safe over the long term. The other question was whether it would measure IOP in a comparable manner to tonometry, and those studies essentially showed that the measurements were accurate. Sometimes there were discrepancies, for which they don’t always have an explanation, between the device and Goldmann applanation.”
The Eyemate is CE-marked and cleared for commercialization in the EU; and Dr. Mansouri, who consults for the company, hopes it will become available on the market soon. “It is being used in clinical trials with several select research centers primarily in Europe, and hopefully in the future, in other parts of the world as well,” he says.
A Low-Cost Concept
At Florida International University, S.S Iyengar, PhD, Ryder Professor of Computer Science and director of computer and information science, is seeking investors to test and refine a color-changing IOP monitoring chip that sits between the cornea and the inferior iris, implanted through a minor in
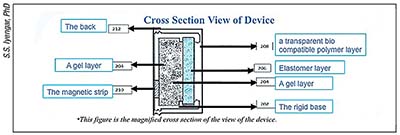 |
| Cross section of Dr. Iyengar’s intraocular IOP-monitoring device, which is made of elastomer and gel layers on a rigid base. The elastic layers expand across a fixed background to produce color change as IOP increases. |
cision. The as-yet unnamed device consists of two pieces: the implant, which is 1 mm tall, 2 mm long, and 1 mm deep; and a handheld binocular viewing device weighing 10 oz. The chip, made of elastomers and an inner hydrogel layer, is visible up close in the eye and appears to change color in response to intraocular pressure changes.
“Presently there are many methods of measuring intraocular pressure, but this is an implantable device with pressure sensors based on electrical resistance,” says Dr. Iyengar. He believes that his user-friendly device could be produced very cheaply. “The pattern in one diffraction grating will change due to the interference effect of light,” he explains. “The pattern is a hologram that changes to contrast with the background pattern and the rigid base, which remains constant. A perceived color change serves to indicate changes in the IOP level.” The viewer and chip need no outside energy source. The viewer consists of magnifiers and mirrors, and a color key that lets patients grade their IOP level. When the chip appears blue, the patient’s IOP is acceptable; magenta means caution; red means that pressure is unacceptably high and the patient should seek ophthalmic care.
Like the Triggerfish lens, this intraocular sensor doesn’t measure absolute IOP. Blue color represents zero; color changes indicate gradations of IOP, theoretically allowing patients at risk of glaucoma to know at a glance when to seek medical attention. Dr. Iyengar says that established glaucoma patients could determine how well their current treatment regimen is working to stabilize their IOP. He wants to further refine his invention, however. “We have very broad levels of distinction of IOP now, indicating the best, the baseline and the worst IOPs. In order to become more precise, what we really want to do is make 150 to 200 of these devices to test in rabbits, eventually leading to very precise readings as to what level we can consider the threshold that reaches glaucoma,” he says. Dr. Iyengar would also like to develop the device as a contact lens.
“I’m not interested in making money,” he adds. “I’m originally from India. I saw a lot of people get glaucoma and by the time they reached about 45 or 50 years old, they lost their eyesight. That was a really strong motivator to come up with something. I came up with this idea with a few friends and tested it. I’ve spent around $10,000 to $15,000 of my own money so far to get the U.S. patent. Now I’m looking for some investors. My goal is to help financially disadvantaged people everywhere in the world by donating the technology. I’m very passionate about this because I want to give back to economically disadvantaged people who lose their productive lives to glaucoma.”
How to Use IOP Data?
An ideal round-the-clock IOP monitoring system would be accurate, convenient, easy to use, easily standardized, and low- to no-maintenance. While our current technologies hold great promise, experts say that nothing commercially available checks all of the boxes yet. “The Holy Grail is an implantable monitoring system, and we’re close,” said Dr. Singh at the ASCRS meeting, adding that an implantable transducer-based system could detect “under-the-radar action” of IOP fluctuations frequently missed during sleep or at night. Researchers have been looking for this since their findings have suggested that even glaucomatous eyes with normal in-office IOP measurements are subject to fluctuations in IOP, and that these appear to be independent risk factors in disease progression.9
“There are companies that are working on implantable devices to measure IOP continuously,” Dr. Mansberger says. “I still think the jury’s out about how much it adds. But from an epidemiology perspective, it would seem that having a more comprehensive assessment of intraocular pressure would be important and valuable,” he says.
“In glaucoma, it is wholly insufficient to have one daytime IOP reading every three or six months,” says Dr. Mansouri. “But unfortunately, there is no longitudinal study that looks at the impact of round-the-clock or day and nighttime IOP measurements and their impact on lowering rates of progression and controlling glaucoma. This is something insurance companies will ask for before they provide adequate reimbursement: They’re going to ask for socioeconomic data on the usefulness of these values; and that’s really up to us in the medical community and manufacturers to provide.”
Dr. Mansouri already uses the Icare Home and the Triggerfish with his patients, and says that both devices have helped him with clinical decision-making. “Using both methods has helped some patients to identify whether peak IOPs occurred in the daytime or nighttime. Sometimes, having a more accurate view of the patient’s circadian IOP rhythm can help us make a better choice about drugs or about the timing of drop application. In some patients, it has given us more data to decide to go into surgery, rather than continuing to change the IOP-lowering drop regimen,” he says. “More importantly, we empower patients by giving them either the possibility of obtaining the measurements themselves, or of viewing the data with us to get a visual look at the IOP, helping to make glaucoma a less-abstract disease. This can improve adherence to topical medications.”
Dr. Mansberger, who currently doesn’t use round-the-clock IOP monitoring outside the clinic with his patients, points out that while many ophthalmologists believe these technologies are important, they don’t yet definitively know how to use the data they obtain. “The question is, how much does self-monitoring IOP improve care, prevent blindness or prevent glaucoma’s progression?” he asks. “We don’t have any verdict on that. There are just so many unknown factors in glaucoma.” REVIEW
Dr. Mansouri is a consultant for Implandata and Sensimed AG. Dr. Mansberger reports no financial interests relevant to this article. Dr. Iyengar holds the patent for his device, and can be contacted at iyengar@cis.fiu.edu.
1. Kapetanakis W, Chang MP, Foster PJ, et al. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br J Ophthalmol 2016;100: 86-93.
2. Takagi D, Sawada A Yamamoto T. Evaluation of a new rebound self-tonometer, Icare HOME: Comparison with Goldmann applanation tonometer. J Glaucoma 2017;26:7:613-18.
3. Pronin S, Brown L, Megaw R, Tatham A. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol 2017;135:10:1-7
4. De Moraes CG, Jasien JV, Simon-Zoula S, et al. Visual field change and 24-hour IOP-related profile with a contact lens sensor in treated glaucoma patients. Ophthalmology 2016;123:4:744-53.
5. Downs JC, Burgoyne CF, Seigfreid WP, et al. 24-hour IOP telemetry in the nonhuman primate: Implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci 2011;52:10:7365–7375.
6. Turner DC, Samuels BC, Huisingh C, Girkin CA, Downs JC. The magnitude and time course of IOP change in response to body position change in nonhuman primates measured using continuous IOP telemetry. Invest Ophthalmol Vis Sci 2017;58:14:6232-6240.
7. Jasien, JV, Jonas JB, de Moraes CG, Ritch R. Intraocular pressure rise in subjects with and without glaucoma during four common yoga positions. Plos One 2015; https://doi.org/10.1371/journal.pone.0144505
8. Dunbar GE, Shen BY, Aref AA. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perceptions. Clin Ophthalmol 2017;11:875-882.
9. Asrani S, Zeimer R; Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9:2:134-42.




