In this article, we chronicle ophthalmology’s efforts to overcome these problems and find even better treatments for exudative AMD.
Current Treatment
Bevacizumab, ranibizumab and aflibercept have provided invaluable visual benefit for patients with exudative AMD. In 2006, the ANCHOR and MARINA trials showed that treatment with these agents could provide visual benefit in such patients1,2 and drastically changed physicians’ and patients’ expectations regarding outcomes.
Unfortunately we still have the issue of a costly, ongoing treatment that puts a significant burden on both the patient and the physician. Trials have looked at monthly, PRN, quarterly and, more recently, treat-and-extend protocols for wet AMD, and found that monthly treatment appears to optimize visual outcomes. A study published in 2014, however, reported that patients with exudative AMD receive significantly fewer injections than clinical trials recommend.3 Unfortunately, less-frequent treatment can lead to poorer visual acuity outcomes. The SEVEN-UP study showed us that, seven years after ranibizumab therapy, in the ANCHOR and MARINA trials, one-third of patients had poor visual results. It’s apparent that despite years of therapy, patients are still at significant risk of visual decline and require continued treatment.4
Several approaches are being taken to address the issues of continued vision loss in some patients and the treatment burden of frequent injections. These include finding better anti-VEGF agents, improving anti-VEGF delivery and developing drugs with targets other than VEGF.
Novel Anti-VEGF Agents
One approach to improving treatment of exudative AMD includes development of new anti-VEGF agents. Some of these agents may be able to extend treatment duration, lessening the burden of frequent injections.
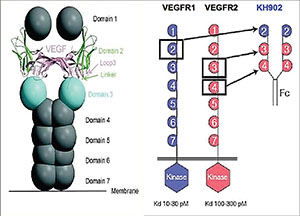 |
| Conbercept, approved in China for AMD treatment, is a fusion molecule combining IgG Fc and VEGF receptors. |
• Brolucizumab. Formerly known as RTH-258 and ESBA-1008, brolucizumab is being developed by Alcon and Novartis. It is a humanized, single-chain antibody fragment that inhibits VEGF, and is able to bind to all isoforms of VEGF-A. Brolucizumab is significantly smaller than bevacizumab, ranibizumab and aflibercept, which may allow for better tissue penetration, more active drug reaching the target tissue and possibly less systemic exposure. Animal studies of brolucizumab and ranibizumab have shown more of the former entering the retina, as well as less systemic drug exposure.5,6
Early human trials suggested that a single injection of brolucizumab may have more potent and longer-lasting effects than a single dose of ranibizumab. The drug showed a reduction in central subfield thickness on OCT comparable to ranibizumab, and there was also a greater mean gain in best-corrected visual acuity compared to ranibizumab, with a trend towards a longer duration of effect.5,6 A subsequent trial showed results similar to aflibercept.7 A Phase III trial is currently ongoing.
• Abicipar. Formerly known as AGN-150998A, abicipar is a DARPin (Designed Ankyrin Repeat Proteins) agent being developed by Allergan. A DARPin is a new class of binding molecule that has a smaller molecular weight and higher target binding affinity than antibodies or antibody fragments.8 Because this agent has a small molecular size and repeat structure, its affinity for VEGF is high.
The Phase II REACH study was designed to analyze abicipar in wet AMD.9,10 In the Phase IIb study, abicipar provided equal or greater vision gains, with the potential for fewer injections, compared to ranibizumab. Phase IIb data suggest that abicipar may be injected every 12 weeks after loading doses. Since the initial results were promising, the Phase III clinical trial started enrollment in July 2015.11
• Conbercept. This is a fusion protein of key domains from human VEGF receptors 1 and 2 with human IgG Fc that’s approved in China for the treatment of AMD.12 It has a high affinity for all VEGF-A/B isoforms as well as placental growth factor. A trial comparing two doses of conbercept showed positive visual acuity outcomes for exudative AMD.13
• OPT-302. Opthea’s OPT-302 is a vascular endothelial growth factor receptor 3 (VEGFR-3), or “Trap,” molecule. This is the first anti-VEGF agent targeted towards VEGF-C and -D. OPT-302 is currently in Phase I dose-escalation trials both by itself and as an agent used in combination with ranibizumab. Enrollment is complete.14
Gene Therapy to Impact VEGF
Another method to improve drug therapy for patients with neovascular AMD is to develop a better anti-VEGF delivery system. One such avenue is gene therapy.
Gene therapy involves introducing genetic material into cells in order to compensate for abnormal or absent genes. The goal of gene therapy is to allow for the production of a beneficial protein in cells that otherwise lack that protein. In gene therapy, the gene carrier, called a vector, is genetically engineered to deliver the gene into cells. Currently, the most popular vector is the adeno-associated virus, as viruses, by their very nature, are able to deliver genetic material into another genome. Researchers hope that gene therapy could be used to provide long-term delivery of anti-VEGF therapy to an eye with exudative AMD.
Drugmakers Genzyme and Avalanche are attempting to utilize adenovirus’s innate transfection capabilities to provide long-lasting gene therapy via intravitreal or subretinal administration, respectively. In Genzyme’s approach, it adds the genetic sequence for sFLT01, a tyrosine kinase inhibitor, to the genetic sequence of the adenovirus producing AAV2-sFLT01. AAV2-sFLT01 is injected intravitreally. As an anti-VEGF agent, sFLT01 blocks the VEGF cascade more distal to where the current anti-VEGF agents block it.15
In animal studies, the intravitreal injection of AAV2-sFLT01 led to sFLT01 expression in the anterior chamber fluid of the animals. In addition it was also associated with decreased growth of laser-induced choroidal neovascularization.16 A Phase I, open-label, multicenter, dose-escalating safety and tolerability study of a single intravitreal injection of AAV2-sFLT01 in patients with neovascular AMD is still ongoing, and has completed enrollment.17
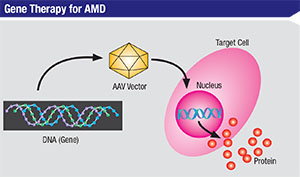 |
| In gene therapy approaches to AMD treatment, adeno-associated virus can be programmed to introduce therapeutic DNA sequences into target cells. |
Other Forms of Delivery
Another method of providing longer duration of drug delivery with the goal of decreasing treatment burden would be to implant a reservoir in the eye that releases medication over an extended period of time. One study, the LADDER trial sponsored by Genentech, is designed to compare a refillable ranibizumab drug port delivery system to monthly ranibizumab injections.22 It’s currently recruiting patients.
Identifying Additional Targets
Developing drugs aimed at therapeutic targets other than VEGF offers another opportunity for improving treatment of exudative AMD. Fortunately, in addition to VEGF, there are many other compounds involved in angiogenesis. Targeting these other compounds may improve treatment of choroidal neovascularization by attacking CNV from different angles.
• Platelet-derived growth factor. This is a growth factor whose production is induced by hypoxia, and it’s also been implicated in angiogenesis. Overproduction of PDGF by endothelial cells increases pericyte content of vessels, leading to the formation of mature vascular networks.23 Disruption of the VEGF pathways with anti-VEGF agents can induce upregulation of PDGF, which in turn allows pericytes to make vascular networks more mature and, therefore, more resistant to further anti-VEGF treatment.24-26 Additionally, overactivity of PDGF has been associated with the development of fibrosis.27 In oncology literature, anti-PDGF agents disrupt pericyte-endothelial communication, sensitizing the neovascular tissue to anti-VEGF agents.28 Theoretically then, an anti-PDGF therapeutic agent could remove the pericyte protective network from the neovascular lesion, exposing the underlying endothelial cells to the anti-VEGF agent. This approach may allow for neovascular regression instead of the current method of disease maintenance. Multiple companies are developing anti-PDGF agents, including Ophthotech, Santen, Allergan and Regeneron.
Fovista is a pegylated aptamer developed by Ophthotech.29,30 Phase IIb data has shown that Fovista, when combined with ranibizumab, improves visual outcomes compared to ranibizumab monotherapy. When comparing 0.3 mg to 1.5 mg of Fovista combined with ranibizumab versus ranibizumab alone, at the higher Fovista dose, the combination therapy improved visual acuity by 62 percent over baseline. Patients were 71 percent (0.3-mg dose) and 190 percent (1.5-mg dose) more likely to experience greater than four and five lines of visual improvement with Fovista. Fovista combined with ranibizumab resulted in a 119 percent relative benefit towards achieving a visual acuity of 20/25 or better. In addition to improvement in intraretinal, subretinal and sub-RPE fluid, there was reduction in subretinal hyperreflective material with combination therapy. Researchers are hoping that the antifibrotic nature of Fovista will lead to reduced fibrosis and vision loss in patients treated with combination therapy vs. monotherapy.31-33
The phase III studies are currently in progress for Fovista in combination with ranibizumab for the treatment of exudative AMD. The hope is that the inhibition of both VEGF and PDGF may result in a reduction of exudation from CNV as well a reduction of the neovascular complex itself. The antifibrotic effect of anti-PDGF agents may also result in less scarring in patients and therefore may have a significant positive impact on visual acuity.
• DE-120. Santen’s DE-120 is a molecule that acts as a dual-kinase receptor inhibitor and is able to block both VEGF and PDGF.34 The Phase I/II study is an open-label, dose-escalating, sequential-cohort study of DE-120 injectable solution administered in patients with late-stage exudative AMD. The study is ongoing but is no longer recruiting new patients.34
• OHR-102 (squalamine). What makes this agent from Ohr Pharmaceutical unique is that it’s topicallyadministered, which would offer a less-invasive way to treat exudative AMD.
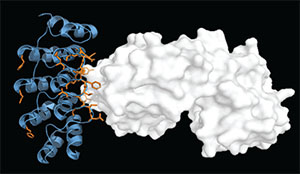 |
| Designed ankyrin repeat proteins (DARPins) like those used in abicipar have a small molecular size with a repeat structure that can be created for a specific duration. |
Squalamine is an amino sterol developed from dogfish shark cartilage. It’s taken up by endothelial cells and is a potent anti-angiogenic molecule that binds calmodulin intracellularly, preventing receptor activation and causing broad inhibition of multiple factors including VEGF, PDGF and basic fibroblast growth factor.10 Phase II trials of topical squalamine combined with intravitreal ranibizumab vs. intravitreal ranibizumab alone showed visual acuity improvements and reductions in retinal thickness on OCT.10 Patients in the phase II IMPACT study of squalamine who had classic-CNV-containing lesions experienced an acuity improvement of +11 letters with squalamine in combination with ranibizumab versus +5 letters with ranibizumab alone. Three-line gains in visual acuity were present in 44 percent of these patients in the combination treatment arm compared to 29 percent in the ranibizumab monotherapy arm.35 A Phase III trial is planned.
• Pazopanib, Regorafenib and PAN-90806. Pazopanib is another medication administered topically, and is being developed by GlaxoSmithKline. It’s a tyrosine kinase inhibitor that inhibits VEGF receptors and the PDGF pathway.
Regorafenib (Bayer AG) is another tyrosine kinase inhibitor that inhibits VEGF receptors, TIE2 and other tyrosine kinase receptors. Finally, PAN-90806 (PanOptica) is a topical drop that acts as a selective VEGF inhibitor.
While our current approach to treating AMD has had a positive impact on visual acuity, it has limitations that must be addressed: Patients still can experience vision loss and there remains a significant treatment burden. Help appears to be on the way, however. Longer-acting drugs, new methods of sustained delivery and novel compounds that target other agents involved in angiogenesis besides VEGF will help improve outcomes. REVIEW
Drs. Levison and Barakat practice with Retinal Consultants of Arizona in Phoenix. Dr. Dugel is managing partner of Retinal Consultants of Arizona, clinical professor at USC Roski Eye Institute, and executive director of the Phoenix Eye Institute.
Dr. Levison has no pertinent financial interests. Dr. Barakat reports financial interest in Ohr as a minor investor. Dr. Dugel reports financial interests as a consultant to Alcon, Allergan, Acucela, Avalanche, Genentech, Novartis and Regeneron, as well as an investor/consultant in Ophthotech. Contact Dr. Levison at 1101 E Missouri Ave, Phoenix, AZ 85014 or ALevison@retinalconsultantsaz.com.
1. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:14:1432-44.
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:14:1419-31.
3. Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol 2014;157:4:825-33.
4. Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:11:2292-9.
5. Dugel P. Results of ESBA 1008, a single-chain antibody fragment, for the treatment of neovascular AMD. ASRS Annual Meeting. San Diego, 2014.
6. Holz FG, Dugel PU, Weissgerber G, et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular AMD: A randomized controlled study. Ophthalmology 2016 doi: 10.1016/j.ophtha.2015.12.030[published Online First: Epub Date]|.
7. Positive phase II data highlights benefits of Alcon’s RTH258 for patients with neovascular (wet) age-related macular degeneration. https://www.novartis.com/news/media-releases/positive-phase-ii-data-highlights-benefits-alcons-rth258-patients-neovascular. Accessed 29 July 2016.
8. Pugach P, Krarup A, Gettie A, et al. In vivo binding and retention of CD4-specific DARPin 57.2 in macaques. PLoS One 2010;5:8:e12455 doi: 10.1371/journal.pone.0012455[published Online First: Epub Date]|.
9. Evaluation of AGN-150998 in exudative AMD. https://clinicaltrials.gov/ct2/show/NCT01397409. Accessed 29 July 2016.
10. Pecen PE, Kaiser PK. Current phase 1/2 research for neovascular age-related macular degeneration. Curr Opin Ophthalmol 2015;26:3:188-93.
11. Abicipar, http://www.molecularpartners.com/our-products/abiciparandmulti-vegf_pdgf/. Accessed 29 July 2016
12. Nguyen TT, Guymer R. Conbercept (KH-902) for the treatment of neovascular age-related macular degeneration. Expert Rev Clin Pharmacol 2015;8:5:541-8.
13. Li X, Xu G, Wang Y, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 2014;121:9:1740-7.
14. Ophthea completes patient enrollment for OPT 302 Phase 1 dose escalation trial in Patients With Wet AMD. http://www.marketwired.com/press-release/opthea-completes-patient-enrolment-opt-302-phase-1-dose-escalation-trial-patients-with-asx-opt-2110458.htm. Accessed 29 July 2016.
15. Pechan P, Rubin H, Lukason M, et al. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther 2009;16:1:10-6.
16. Lukason M, DuFresne E, Rubin H, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther 2011;19:2:260-5.
17. Safety and tolerability study of AAV2-sFLT01 in patients with neovascular age-related macular degeneration. https://clinicaltrials.gov/ct2/show/NCT01024998. Accessed 29 July 2016.
18. Lai CM, Shen WY, Brankov M, et al. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther 2005;12:4:659-68.
19. Bennett J, Maguire A, Cideciyan A, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci U S A 1999;96:17:9920-5.
20. Chalberg T. Anti-VEGF gene therapy: Early clinical results using the ocular biofactor in wet AMD. Presented at: Angiogenesis, Exudation, and Degeneration. Miami, Feb 8, 2014.
21. Avalanche Biotechnologies Inc announces positive top-line Phase 2a results for AVA-101. http://investors.avalanchebiotech.com/phoenix.zhtml?c=253634&p=irol-newsArticle&ID=2059444
22. Study of the efficacy and safety of the ranibizumab port delivery system for sustained delivery of ranibizumab. https://clinicaltrials.gov/ct2/show/NCT02510794.
23. Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999;126:14:3047-55.
24. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:5706:58.
25. Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997;277:5323:242-5.
26. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:7070:967-74.
27. Greenberg JI, Shields DJ, Barillas SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008;456:7223:809-13.
28. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:10:1276-312.
29. Jaffe GJ, Eliott D, Wells JA, et al. A Phase I study of intravitreous E10030 in combination with ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2016;123:1:78-85.
30. Tolentino MJ, Dennrick A, John E, Tolentino MS. Drugs in Phase II clinical trials for the treatment of age-related macular degeneration. Expert Opin Investig Drugs 2015;24:2:183-99.
31. Dugel PU. Phase IIb clinical trial of Fovista anti-PDGF therapy in patients with neovascular AMD. Presented at: American Academy of Ophthalmology annual meeting. Chicago, November 2012.
32. Chakravathy U JG. Dual antagonism of platelet derived growth factor and vascular endothelial growth factor results in reduced subretinal fibrosis and neovascular growth. Presented at: American Academy of Ophthalmology annual meeting. New Orleans, October 2014.
33. Dugel PU. Anti-platelet derived growth factor: Where do we stand? American Academy of Ophthalmology annual meeting. Chicago, November 2012.
34. A Phase I/II safety study of DE-120 injectable solution for AMD. https://clinicaltrials.gov/ct2/show/NCT02022501.
35. Ohr Pharmaceutical data from OHR-102 phase II IMPACT Study in wet-AMD. Data on file, Ohr Pharmaceutical.



