Descemet’s membrane endothelial keratoplasty is the selective transplantation of the Descemet’s membrane and endothelium and is currently one of the most common surgical treatments for corneal endothelial dysfunction. Despite the already advanced status of the surgical procedure, DMEK is continuously being optimized to achieve positive outcomes, including a good functional outcome, relatively rapid visual recovery, low risk of hemorrhage and infection, reduced astigmatism, decreased corneal denervation and decreased rejection rates.1 However, challenges of DMEK have been reported in the preoperative graft preparation, and complications may arise in the initial post-transplant weeks and are most evident at the initial postoperative clinic examination.2 The diagnosis and successful management of these early complications are essential for the long-term survival of DMEK.3 Studies show that the complication rate decreases significantly with DMEK surgeons’ growing experience over the years.4 Understanding the surgical technique and various nuances of DMEK surgery can be very helpful in obtaining an optimal result.5
In this article, we discuss the diagnosis and management strategies for complications of DMEK that occur intra- and postoperatively to achieve the goals of performing the procedure safely, reducing complications and making the procedure simpler for ophthalmic surgeons to reproduce.
Donor Tissue
The goal of corneal, surgical endothelial intervention is to usually replace the dysfunctional endothelial cells with healthy donor tissues and to provide optimal vision for the patient.
The donor tissue scrolls for DMEK surgery have been used in two varieties namely, endothelium-out and endothelium-in (trifold) orientation.6 The outcomes being similar, it may then be a surgeon preference.6
Donor age can contribute to different behavioral patterns with regard to tissue scrolling and unscrolling, all of which can affect the surgical duration and intraoperative complexities of DMEK. When we look at donor age, the younger the donor, the more elastic the DM lamella, which allows the graft to coil up disproportionately, making unfolding in the anterior chamber, and fixing the graft to the recipient corneal bed, surgically challenging. Young donors have been found to scroll tighter than old donors.7 Hence, it appears that an elderly donor is more suitable for unscrolling an endothelium-Descemet’s membrane (EDM) disc, especially in eyes with a very deep or very shallow AC, that makes transplantation difficult. However, some older donor graft tissue may not scroll optimally and lead to additional surgical difficulties intraoperatively.8
 |
|
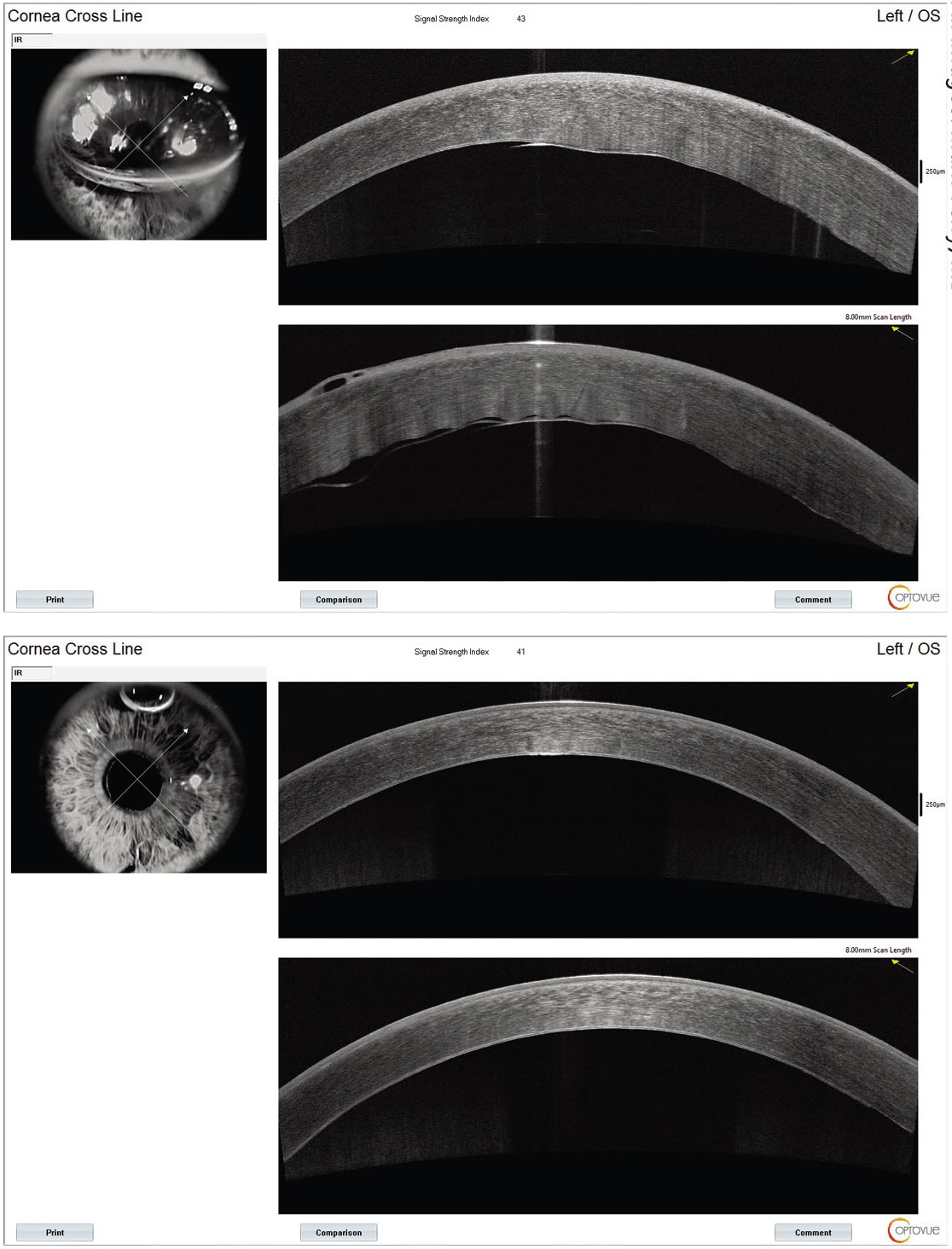
Figure 1. (Top) Optical coherence tomography image of a donor EDM disc detachment treated with re-bubble. (Bottom) Same eye five days after rebubble, showing optimal attachment of DMEK donor graft and almost complete resorption of the air bubble. |
Incorrect orientation of the EDM disc (endothelial-side-up) intraoperatively requires graft detachment by aspirating the AC air bubble and injecting balanced salt solution from a side port, and then injecting air or gas to reattach it in its proper orientation namely, endothelial-side-down. Postoperative detection of an upside-down DMEK requires timely surgical intervention8 to establish correct orientation and re-establishment of the tissue anatomy between the donor and recipient tissues.
When comparing complication rates between younger and older donors, one study showed that younger donor age had comparable clinical outcomes and no difference in complication rate to older donor age in DMEK surgery within the first postoperative year.9 It’s also been shown that donor age and endothelial cell density influence the properties of the DMEK grafts. Older donors and grafts with higher endothelial cell densities formed broader graft rolls.10
In addition to donor age and endothelial cell density, tissue characteristics such as the diameter of the graft, storage methods, methods of transplantation, and the recipient’s AC status have also been reported as determining factors in the scrolling and unscrolling/unfolding of the DMEK graft. Therefore, it’s important to consider these and other variables when optimizing for DMEK surgery.
Decreasing EDM disc unfolding time within the anterior chamber facilitates DMEK surgery and decreases potential iatrogenic donor endothelial cell loss. Pre-positioning the donor EDM disc prior to AC delivery of the donor tissue often facilitates easy and quick unfolding of EDM disc and shortens DMEK surgical time.11
While trypan blue greatly facilitates viewing the EDM disc within the recipient AC, it appears to have an effect on the DM elasticity. Trypan blue may decrease DM elasticity and consequently increase its stiffness.12
Challenges of Preparing a DMEK Graft
It’s been noted that donor preparation problems can occur in individuals with systemic diseases such as diabetes mellitus, hyperlipidemia and obesity.13 This may be due to glycosylation products, and oxidative stress may have induced increased cross-linking of corneal collagen fibers, resulting in ultrastructural factors such as difficulty separating adhesions between the stroma and DM.
Larger graft size has been recommended in patients with dysfunction of central and peripheral endothelium due to pseudophakic bullous keratopathy.14 However, there’s no definitive recommendation regarding the optimal graft size for DMEK, after failed penetrating keratoplasty. Some propose undersizing the DMEK graft diameter by 0.25 to 0.50 mm relative to the previously failed PK graft diameter,15 whereas others find no difference in outcomes between oversized, same-sized and undersized DMEK graft diameters.16
Anterior chamber depth and posterior corneal profile have to be taken into account when sizing the DMEK graft in order to facilitate graft unfolding, graft attachment, and reduce endothelial cell (EC) loss.17 A small graft can accommodate patients with small eyes, such as those with high hyperopia or a narrow AC, whereas a graft larger than 9.5 mm can accommodate patients with myopia and buphthalmic eye or a deep AC.18 Patients who have previously undergone glaucoma drainage device surgery may benefit from a small graft size, not only to facilitate the unfolding and attachment of the graft but, more importantly, to avoid contact with the drainage tubing.19
Preparing a graft smaller than 5 mm or larger than 9 mm can be somewhat demanding. The difficulties of a small DMEK graft preparation include peeling, marking, loading and unfolding. Although multiple confounding factors have been reported to influence graft scrolling,20 small grafts without enough peripheral area may not roll and stay flat. Specifically, a small-diameter graft often doesn’t have enough area to be folded to maintain the tissue architecture in the preloaded DMEK device. Pre-loading EDM DMEK tissue with endothelium facing inwards (DM-endothelium complex folded as taco-like tissue with endothelium facing inwards) can be challenging, and the graft can lose orientation.21 In contrast, an endothelium-outward, pre-loaded small EDM DMEK graft could be more feasible.6
A large-diameter graft may be needed in some DMEK cases as mentioned above. Corneal EC density is higher in the paracentral and peripheral regions compared with the central cornea.22
When preparing large-diameter DMEK grafts, peripheral or horseshoe-shaped tears are potential risks, as fragile tissues can tear at the periphery while stripping. One approach to handle an accidental peripheral tear is to make a decentralized, relatively smaller graft inside the large-diameter grafts after stripping.23 The advantage of harvesting a decentralized graft of similar size is that it also provides more EC from the periphery. As the corneal EC density is higher in the periphery, transplanting larger or decentered grafts could potentially provide more EC to increase overall graft survival. It’s also important to avoid donors with previous cataract incision wounds at the mid-periphery of the cornea or with deep corneal scars. Similarly, a decentralized, relatively smaller-diameter graft can be obtained to avoid tissue wastage and provide a larger number of EC for transplantation.
The Keys to Performing A Descemetorhexis
A crucial step in DMEK procedures is descemetorhexis, the removal of the recipient’s diseased DM and EC, before transplantation. Poor visualization of the recipient DM during descemetorhexis can result in too small or incomplete recipient DM removal, whereas a same-size or slightly larger descemetorhexis compared with the implantable graft diameter is the most desirable. Application of ophthalmic viscosurgical devices (OVDs) like Healon, trypan blue staining, and air in the AC have been reported to allow a better operative view.24
 |
|
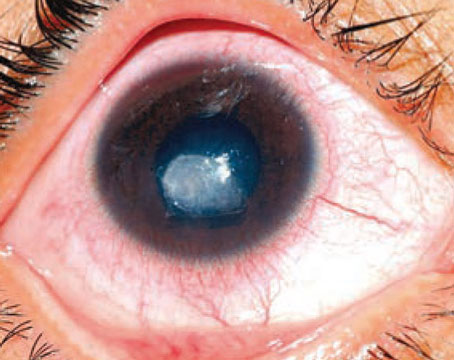
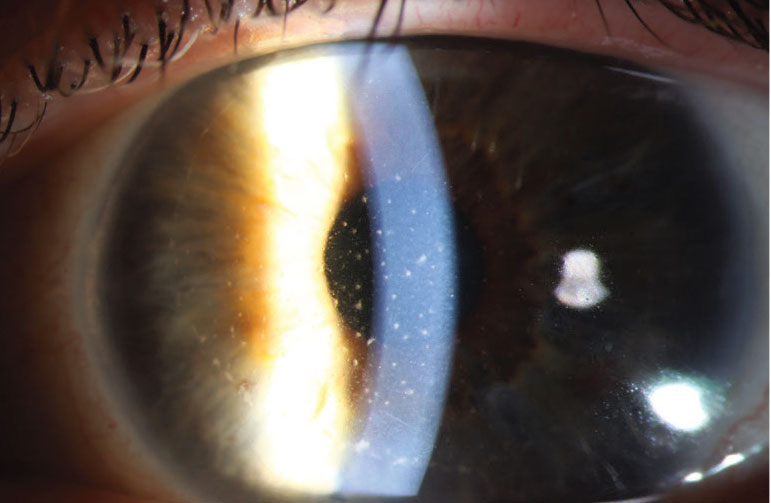
Figure 2. Slit-lamp photograph showing keratic precipitates in a DMEK graft, one year after DMEK surgery. Note that the KP are only on the area covered by the DMEK graft and aren’t found outside of the graft edges, indicating that the area outside the edges is covered by recipient endothelium. |
Descemetorhexis techniques such as initiating from the central area followed by enlarging it similar to a capsulorhexis or utilizing femtosecond laser have been proposed to prevent incomplete descemetorhexis. However, the paucity of comparative studies makes it difficult to determine the most effective method for descemetorhexis.
One of the important factors to keep in mind while performing a mechanical descemetorhexis is to maintain the proper plane of dissection with the blunt-tipped instrument without entering the recipient corneal stroma. If inadvertent stromal entry occurs, the instrument tip will usually be temporarily stuck in that location or cause increased tissue resistance. In such cases, it’s best to withdraw, and then re-establish the proper plane to continue and complete descemetorhexis. Avoid the temptation to use additional force to overcome the resistance in such cases. Keeping the inner stromal surface of the recipient cornea in its pre-surgical state contributes to a better overall donor-recipient interface and potentially better vision.
Challenges of DMEK Graft Delivery
Proper delivery and implantation of donor graft in the AC of the host is important to prevent donor tissue injury.
Various unfolding techniques are often based on the characteristics of the DMEK scroll in the AC, and most grafts are between 8 and 8.5 mm. Delivering and unfolding grafts sized more than 8 mm is reportedly challenging. However, they have the advantage of being easier to centralize due to their relatively large diameter. In contrast, small grafts are relatively more difficult, especially in cases with deep ACs. The AC needs to be kept shallow during all surgical maneuvers related to EDM disc unfolding.25
In general, the unfolding can be achieved through indirect manipulation with the aid of air, fluids, gentle tapping and sliding of cannulas on the outer corneal surface.
Specifically, the graft delivery and unfolding procedures can be somewhat complex in eyes that have undergone glaucoma surgery, especially those involving a glaucoma drainage tube.26 Contact between the graft and the tube must be avoided to reduce the risk of graft detachment and EC loss. Also, injected air can partially escape through the opening in the seton tube within the AC, making the surgical procedure more difficult. Also, the graft has to unfold and attach above the tube, to the recipient inner corneal stroma. Also, repeated air injections are recommended to fill the AC adequately and pressurize the eye for an average of more than an hour, thereby reducing the rate of graft detachment.26 Importantly, patent peripheral iridectomy should be done, preferably at the 6 o’clock position, to prevent potential pupillary block.
The unfolding and fixation of the EDM disc can be difficult in patients who are post-vitrectomy, have AC or iris-clip intraocular lens implants, or are aphakic or aniridic. Rarely, the donor DMEK graft tissue can pass through the pupil and land in between a PC IOL and an intact posterior lens capsule, giving the IOL a bluish appearance due to the trypan blue stained DM behind the PC IOL. When this occurred to one of the authors (TJ), no-touch fluidics was able to bring the DMEK graft back into the AC and it was attached to the recipient cornea with very good visual outcome.
The oil in a silicon-filled vitrectomized eye can get into the AC and impede the unfolding of the graft, which is associated with an increased risk of graft detachment.
In aphakic or vitrectomized eyes with a scleral- or iris-fixed intraocular lens, the injected air bubble can easily get behind the iris and lead to angle closure or even graft dislocation into the posterior segment. In such cases, the use of safety hammock sutures may be considered, which one of this article’s authors (TJ) described at the 2017 meeting of the American Society of Cataract and Refractive Surgery in Los Angeles.
When the graft isn’t visualized because of the cloudy cornea, gentle corneal epithelial scraping can improve the surgical view. Postoperative epithelial care is important to reduce the risk of infection and pain, and to achieve good visual results early on in the postoperative period.
Clinical Complications
Following are the complications to watch out for postoperatively in DMEK cases:
• Acute glaucoma. DMEK carries a risk of postoperative IOP elevation due to pupil blockage caused by the air or gas bubble in the AC, or angle closure due to anterior iris displacement caused by bubble migration to the posterior chamber.
A postoperative IOP rise occurs most frequently within the first two hours following DMEK. Such an increase in pressure may result in Urrets-Zavalia syndrome, characterized by iris sphincter muscle ischemia and atrophy. Temporary IOP elevation following surgery doesn’t appear to influence functional or morphological outcomes, however.
To help counter possible risk of pupillary block, you can perform a laser iridotomy prior to DMEK surgery in select cases. Typically, an inferior PI is performed intraoperatively to prevent pupillary block. If the PI isn’t performed or if the air bubble in the AC is so large that it blocks the iridotomy, acute glaucoma attack remains a risk. The risk of pupillary block is greatest during the first 24 hours after surgery, before the bubble in the AC spontaneously absorbs.27
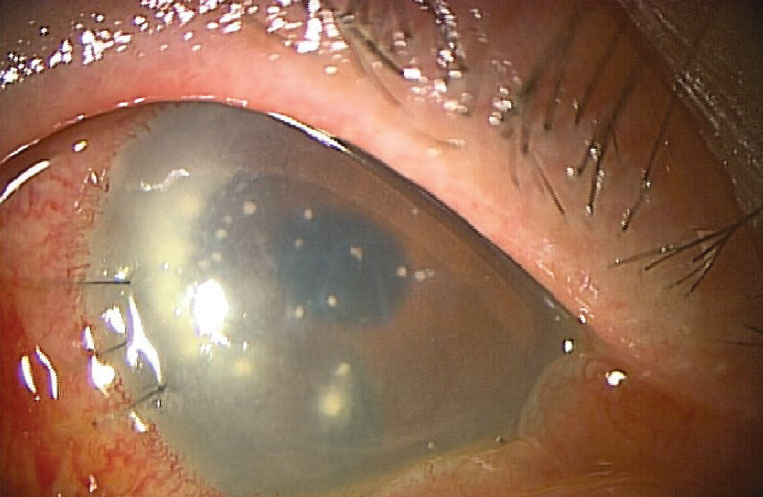 |
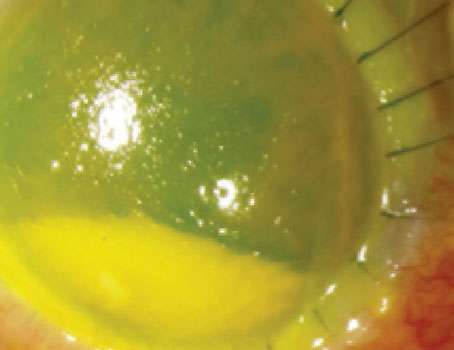
Figure 3. Candida infection of the interface following DMEK surgery. A penetrating keratoplasty and medical treatment resulted in an overall good outcome. |
If the IOP is elevated due to posterior migration of the air bubble, air often can be displaced from the posterior chamber into the anterior chamber using a topical cycloplegic and mydriatic agent.
If pupillary block occurs, where the air bubble in the AC appears to cover the inferior PI, create a paracentesis with a 30-gauge needle on a 1-ml syringe to remove enough air and relieve the pupillary block.
• Endothelial graft detachment. Graft detachment is one of the most common complications after DMEK (Figures 1A and 1B). The detachment can be partial or total, depending on the contact area between the donor tissue and the recipient bed. The reported incidence of partial graft detachment ranged from 4 to 95 percent, with a rebubbling rate between 2.4 and 82 percent, whereas the incidence of total graft detachment is between 0.73 and 7 percent.28
Risk factors reported for graft detachment include graft preparation, learning curve, graft size, age, failed PK and endothelial keratoplasty (EK), history of glaucoma surgery (both glaucoma drainage device and filtrating surgery) and hypotony.
Pseudophakic bullous keratopathy has shown a higher rate of graft detachment compared with Fuchs’ endothelial corneal dystrophy; however, the results of a three-year follow-up showed no statistically significant differences.
DMEK can be safely combined with cataract surgery or operated on sequentially, as graft detachment rates are similar. Abnormal anterior segment anatomy with peripheral synechiae, microphthalmos, aniridia or aphakia patients experienced high detachment and failure rates after DMEK.29,30 SF6 20% as AC tamponade for DMEK significantly reduced the rebubbling rate and was associated with a lower rate of graft detachment, especially inferiorly and mid-peripherally, compared with 100-percent air. 31 However, the long-term visual and IOP results are comparable for both types of AC tamponade techniques. A large descemetorhexis with flat graft detachments can settle down over time, while a small descemetorhexis with a coiled graft detachment usually shows no improvement.32
A slit-lamp examination can diagnose post-DMEK detachment, which is typically characterized by the separation of the graft from the posterior recipient stroma. However, in some eyes, it may also be characterized by persistent corneal stroma edema over the area of DMEK graft detachment but clearing elsewhere. Optical coherence tomography and Scheimpflug imaging are useful for identifying graft detachment following DMEK. OCT can be a useful tool for pre- and postoperative corneal assessment to enhance the orientation assessment and increase the safety of the DMEK procedure, particularly in patients with poor graft visualization.
Most post-DMEK detachments are partial and nonprogressive; therefore, intervention may not be unnecessary. The postoperative treatment depends on the extent and location of the detachment. Numerous reports of spontaneous resolution of corneal edema, either through spontaneous reattachment of the graft Descemet’s membrane or migration of proximal endothelial cells, have been published with promising visual outcomes.33,34 However, detachments located in the center or involving the visual axis, comprising one-third of the graft’s surface area, scrolled-in configuration, should be treated with repeated air or gas injection. The rebubbling procedure should be prompt in order to expedite visual rehabilitation and prevent wrinkling, fibrosis and contraction of the graft.
Despite the fact that the use of 20% SF6 as a tamponade is associated with fewer rebubbling procedures than air,35 one study indicates that 10% C3F8 is an alternative when air or SF6 rebubbling fails.36
Animal studies show that all three gases exhibit a similar endothelial toxicity profile. Reattachment of the graft is between 79 to 92 percent of the time following rebubbling. If rebubbling isn’t performed, corneal clearance typically occurs over a prolonged period of time (mean of six months), and only half of these eyes achieve 20/40 vision. Therefore, if the graft detaches but the cornea doesn’t clear within a month, it’s recommended to conduct a rebubbling. 37 Rebubbling can be approached in the inferior temporal quadrant or at a site where the DM is still attached. There are no reported differences in EC density in patients with single rebubbling or without rebubbling, whereas multiple rebubbling has a higher rate of EC loss.38
A complete detachment means that the post-DMEK graft has completely separated from the posterior corneal surface of the recipient and floats freely in the AC. The entire cornea remains edematous. It may be necessary to remove and replace the graft, or use a rescue technique as an alternative. The rescue technique involves staining the graft in the AC with trypan blue, performing a 20-gauge paracentesis and administering air promptly to prevent staining of the host stroma and to facilitate donor tissue attachment.
If the graft is completely attached after one week, then it usually doesn’t detach. If any detachment is detected one hour after surgery, the patient should be carefully evaluated during the immediate postoperative period; if the detachment persists, it’s unlikely that spontaneous reattachment will occur. If, however, no detachment is observed at one week postoperatively but not at one hour post-DMEK, spontaneous re-attachment is probable in nearly 90 percent of cases.39
• Graft rejection and Descemet’s folds. One study showed that an endothelial immune reaction (“rejection”) (Figure 2) over a period of four years occurs with a probability of around 2.3 percent, particularly in patients without long-term topical cortisone therapy, and that long-term administration of topical steroids is therefore recommended.40 The range of graft rejection is about 1.4 to 5 percent, with an average of about 3.7 percent.41 Since the immune reactions are often clinically asymptomatic, regular follow-up checks should be carried out at least in the first two years to detect subclinical immune reactions. Descemet’s graft folds can occur in 1.9 percent of cases.42
Less Common Complications
The following complications are possible, but less likely than those described above:
• Infection. Rarely, bacterial and fungal interface keratitis can occur after DMEK surgery (Figure 3).43,44 Medical as well as appropriate, timely surgical management are important for preserving vision and optimizing outcomes.
• Other complications. The risk of pupillary block, hypotony, interface pigment deposits, subepithelial haze and anterior synechiae has been reported to be less than 1 percent.42
In conclusion, DMEK surgery provides a near-optimal anatomic procedure for endothelial transplantation in corneal endothelial dysfunction. It can provide the best visual outcome with the lowest endothelial graft rejection rate as compared to all other forms of corneal transplantation for corneas with compromised endothelium.
The continuous improvement process and our increased experience with the procedure lead to the optimization of the postoperative result and minimization of intra- and postoperative complication rates.
Dr. John is in private practice in the Chicago area, and is a clinical associate professor at Loyola University at Chicago.
Dr. Saddemi is a first-year ophthalmology resident at Broward Health in Ft. Lauderdale, and is affiliated with the Specialty Retina Center in Coral Springs, Florida.
Dr. Tighe is affiliated with the Ocular Surface Center in Miami, the Department of Biochemistry and Molecular Biology at the Miller School of Medicine at the University of Miami, and Florida International University’s Herbert Wertheim College of Medicine in Miami. He’s a research scientist at Tissue Tech.
Dr. Cheng is a voluntary clinical associate professor at Florida International University, a research associate at the Miami VA and a clinical scientist at the Ocular Surface Center.
None of the authors have a financial interest in any product mentioned.
1. Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: Safety and outcomes. Ophthalmology 2009;116:9:1818-1830.
2. Romano D, Aiello F, Parekh M, et al. Incidence and management of early postoperative complications in lamellar corneal transplantation. Graefe’s Archive for Clinical and Experimental Ophthalmology. April 27, 2023 (online article). doi:10.1007/s00417-023-06073-6
3. Greenrod EB, Jones MNA, Kaye S, Larkin DFP. Center and surgeon effect on outcomes of endothelial keratoplasty versus penetrating keratoplasty in the United Kingdom. Am J Ophthalmol 2014;158:5:957.
4. Schrittenlocher S, Schaub F, Hos D, Siebelmann S, Cursiefen C, Bachmann B. Evolution of consecutive Descemet membrane endothelial keratoplasty outcomes throughout a 5-year period performed by two experienced surgeons. Am J Ophthalmol 2018;190:171-178.
5. Chen SY, Terry M. Step-by-step Descemet’s membrane endothelial keratoplasty surgery. Taiwan J Ophthalmol 2019;9:1:18-26.
6. Price MO, Lisek M, Kelley M, Feng MT, Price FW. Endothelium-in versus endothelium-out insertion with Descemet membrane endothelial keratoplasty. Cornea 2018;37:9:1098-1101.
7. Bennett A, Mahmoud S, Drury D, et al. Impact of donor age on corneal endothelium-Descemet membrane layer scroll formation. Eye & Contact Lens: Science & Clinical Practice 2015;41:4:236-239.
8. Bardan A, Goweida M, El Goweini H, C Liu C. Management of upside-down Descemet membrane endothelial keratoplasty: A case series. J Curr Ophthalmol 2020;32:2:142.
9. Schaub F, Enders P, Zachewicz J, et al. Impact of donor age on Descemet membrane endothelial keratoplasty outcome: Evaluation of donors aged 17–55 years. Am J Ophthalmol 2016;170:119-127.
10. Heinzelmann S, Hüther S, Böhringer D, Eberwein P, Reinhard T, Maier P. Influence of donor characteristics on Descemet membrane endothelial keratoplasty. Cornea 2014;33:6:644-648.
11. John T, Tighe S, Sheha H. Donor Descemet’s membrane positioning prior to anterior chamber delivery in DMEK surgery. American Society of Cataract and Refractive Surgery Annual Meeting. Published online May 20, 2020.
12. John T, Patel A, Vasavada A, et al. Effect of trypan blue on Descemet membrane elasticity. Cornea. 2016;35:11:1401-1403.
13. Vianna LMM, Stoeger CG, Galloway JD, et al. Risk Factors for Eye Bank Preparation Failure of Descemet Membrane Endothelial Keratoplasty Tissue. Am J Ophthalmol 2015;159:5:829-834.
14. Zwingelberg SB, Büscher F, Schrittenlocher S, et al. Long-term outcome of Descemet membrane endothelial keratoplasty in eyes with Fuchs’ endothelial corneal dystrophy versus pseudophakic bullous keratopathy. Cornea 2022;41:3:304-309.
15. Alió del Barrio JL, Montesel A, Ho V, Bhogal M. Descemet membrane endothelial keratoplasty under failed penetrating keratoplasty without host descemetorhexis for the management of secondary graft failure. Cornea 2020;39:1:13-17.
16. Schrittenlocher S, Schlereth SL, Siebelmann S, et al. Long-term outcome of Descemet membrane endothelial keratoplasty (DMEK) following failed penetrating keratoplasty (PK). Acta Ophthalmol 2020;98:7.
17. Quilendrino R, Rodriguez-Calvo de Mora M, Baydoun L, et al. Prevention and management of Descemet membrane endothelial keratoplasty complications. Cornea 2017;36:9:1089-1095.
18. Quilendrino R, Yeh RY, Dapena I, et al. Large diameter Descemet membrane endothelial keratoplasty in buphthalmic eyes. Cornea 2013;32:5:e74-e78.
19. Birbal RS, Tong CM, Dapena I, et al. Clinical outcomes of Descemet membrane endothelial keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol 2019;199:150-158.
20. Parekh M, Ferrari S, Pagano L, Angi M, Gadhvi K, Romano V. Confounding factors influencing the scroll width of Descemet membrane endothelial keratoplasty graft. Indian J Ophthalmol 2021;69:2:461.
21. Busin M, Leon P, D’Angelo S, et al. Clinical Outcomes of preloaded Descemet membrane endothelial keratoplasty grafts with endothelium tri-folded inwards. Am J Ophthalmol 2018;193:106-113.
22. Amann J, Holley GP, Lee SB, Edelhauser HF. Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. Am J Ophthalmol 2003;135:5:584-590.
23. Tenkman LR, Price FW, Price MO. Descemet membrane endothelial keratoplasty donor preparation. Cornea 2014;33:3:319-325.
24. Parekh M, Romano D, Wongvisavavit R, et al. DMEK graft: One size does not fit all. Acta Ophthalmol 2023;101:1.
25. Kruse FE, Laaser K, Cursiefen C, et al. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 2011;30:5:580-587.
26. Birbal RS, Tong CM, Dapena I, et al. Clinical outcomes of Descemet membrane endothelial keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol 2019;199:150-158.
27. Lee JS, Desai NR, Schmidt GW, et al. Secondary angle closure caused by air migrating behind the pupil in Descemet stripping endothelial keratoplasty. Cornea 2009;28:6:652-656.
28. Deshmukh R, Nair S, Ting DSJ, Agarwal T, Beltz J, Vajpayee RB. Graft detachments in endothelial keratoplasty. British Journal of Ophthalmology 2022;106:1:1-13.
29. Romano D, Aiello F, Parekh M, et al. Incidence and management of early postoperative complications in lamellar corneal transplantation. Graefe’s Archive for Clinical and Experimental Ophthalmology. April 27, 2023 (online article).
30. Matthaei M, Schrittenlocher S, Hos D, et al. Zehn jahre Descemet membrane endothelial keratoplasty bei Fuchs-dystrophie. Der Ophthalmologe 2019;116:3:236-242.
31. Siebelmann S, Lopez Ramos S, Scholz P, et al. Graft detachment pattern after Descemet membrane endothelial keratoplasty comparing air versus 20% SF6 tamponade. Cornea 2018;37:7:834-839.
32. Bucher F, Hos D, Müller-Schwefe S, Steven P, Cursiefen C, Heindl LM. Spontaneous long-term course of persistent peripheral graft detachments after Descemet’s membrane endothelial keratoplasty. British Journal of Ophthalmology 2015;99:6:768-772.
33. Couch SM, Baratz KH. Delayed, Bilateral Descemet’s membrane detachments with spontaneous resolution: Implications for nonsurgical treatment. Cornea 2009;28:10:1160-1163.
34. John T, Kuizin G, Kim AJ, Tighe S, Cheng AMS, Sheha H. Spontaneous restoration of corneal clarity after graft displacement following Descemet membrane endothelial keratoplasty. Journal of Cataract and Refractive Surgery Online Case Reports 2016;4:3:49-51.
35. Marques RE, Guerra PS, Sousa DC, et al. Sulfur hexafluoride 20% versus air 100% for anterior chamber tamponade in DMEK: A meta-analysis. Cornea 2018;37:6:691-697.
36. Keshet Y, Nahum Y, Bahar I, Livny E. Anterior chamber rebubbling with perfluoropropane (C3F8) after failed rebubbling attempts for persistent Descemet membrane endothelial keratoplasty graft detachments. Cornea 2019;38:8:976-979.
37. Price FW, Price MO. To intervene or not to intervene: That is the question. Ophthalmology 2015;122:1:6-7.
38. Gundlach E, Pilger D, Dietrich-Ntoukas T, Joussen AM, Torun N, Maier AKB. Impact of re-bubbling after Descemet membrane endothelial keratoplasty on long-term results. Curr Eye Res 2021;46:6:784-788.
39. Dapena I, Moutsouris K, Ham L, Melles GRJ. Graft detachment rate. Ophthalmology 2010;117:4:847-847.e1.
40. Price MO, Scanameo A, Feng MT, Price FW. Descemet’s membrane endothelial keratoplasty. Ophthalmology 2016;123:6:1232-1236.
41. Monnereau C, Bruinsma M, Ham L, Baydoun L, Oellerich S, Melles GR. Endothelial cell changes as an indicator for upcoming allograft rejection following Descemet membrane endothelial keratoplasty. American Journal of Ophthalmology 2014;158:3:485-95.
42. Monnereau C, Quilendrino R, Dapena I, et al. Multicenter study of Descemet membrane endothelial keratoplasty: First case series of 18 surgeons. JAMA Ophthalmol 2014;132:10:1192-8.
43. Augustin VA, Weller JM, Kruse FE, Tourtas T. Fungal interface keratitis after Descemet membrane endothelial keratoplasty. Cornea 2018;37:11:1366-1369.
44. Gunaydin NT, Tanyıldız B, Kandemir B, Simsek S. Infectious interface keratitis after Descemet membrane endothelial keratoplasty. Arq Bras Oftalmol 2022;85:5.