Retinal vascular occlusive disease, which can involve central trunks or branches of the retinal arterial or venous circulation, is a significant cause of ocular morbidity.1,2 Several classic cardiovascular risk factors such as systemic hypertension and smoking are known risk factors for retinal vascular occlusion. Moderately elevated total plasma level of homocysteine (tHcy) was recently shown to be an independent risk factor for cardiovascular disease.3,4 Because of this, some studies have examined the relationship between elevated plasma tHcy and its determinants and retinal vascular occlusive disease.
Homocysteine Metabolism
Homocysteine is a sulphur-containing amino acid produced during the metabolism of the essential amino acid methionine. Methionine comes from dietary animal protein and is involved in the production of important molecules like creatine, DNA, RNA and neurotransmitters. The resultant homocysteine is cytotoxic and must be metabolized by one of two biochemical pathways. If there is excess methionine, homocysteine is metabolized by the transsulfuration pathway, where it is first converted to cystathionine, a reaction catalyzed by the enzyme cystathionine ß-synthase (CBS), and ultimately forms cysteine. Both reactions require vitamin B6 as a co-factor. If methionine levels are low, homocysteine is converted back to methionine via the remethylation pathway. This reaction is catalyzed by methionine synthase, and vitamin B12 and 5-methyltetrahydrofolate are important co-substrates. The remethylation pathway also requires a constant supply of folate and the pivotal enzyme, 5,10 methylenetetrahydrofolate reductase (MTHFR). Precursor reactions in the maintenance of adequate 5-methyltetrahydrofolate levels also require vitamin B6.
Causes of Elevated tHcy Levels
Inherited defects in the enzymes controlling homocysteine metabolism including CBS, MTHFR and methionine synthase will alter its metabolism and result in elevated plasma tHcy levels. Homozygous deficiency of the gene for CBS results in homocystinuria; plasma tHcy levels can exceed 100 µmol/L.
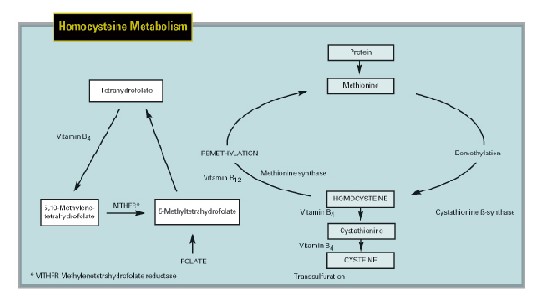 |
| Methionine from dietary animal protein undergoes demethylation and donates methyl groups to vital transmethylation reactions producing creatine and phosphatidylcholine and allows methylation of DNA, RNA and neurotransmitters. The resultant homocysteine is potentially cytotoxic and must be metabolized. When methionine levels are high, homocysteine undergoes transsulfuration with addition of serine to form cystathionine, catalyzed by cystathionine ß-synthase (CBS), which is converted to cysteine. Vitamin B6 is a cofactor for both reactions. When methionine levels are low, homocysteine is remethylated back to methionine by methionine synthase, which requires folate, vitamin B12 and 5-methyltetrahydrofolate and the enzyme, MTHFR. Precursor reactions in the maintenance of adequate 5-methyltetrahydrofolate levels also require vitamin B6. |
Heterozygous deficiency of the CBS gene does not result in homocystinuria, and persons with this defect can have normal fasting plasma tHcy levels. As the CBS enzyme does not function normally, however, administering a large dose of methionine uncovers the less active form of CBS by stressing the transsulfuration pathway with subsequent elevation of plasma tHcy levels. A number of point mutations in the MTHFR gene can result in reduced function of this pivotal enzyme.
The 677C-T mutation is a common mutation that results in a less active, thermolabile form of the MTHFR enzyme. Persons who are homozygous for this mutation have a TT genotype. The prevalence of the TT genotype varies in different populations. The relationship between thermolabile MTHFR and elevated plasma tHcy is unclear, but it is likely linked to serum folate levels. Folate deficiency may lead to expression of the TT genotype, or patients with this genotype may need higher serum folate levels to maintain normal function of the enzyme. Mutations of a gene coding for methylcobalamin synthesis, an essential cofactor of methionine synthase, result in a functional deficiency of the enzyme, leading to increased plasma tHcy and hypomethioninemia.
Dietary factors can alter plasma tHcy levels and excessive intake of methionine–rich animal protein leads to an elevation of plasma tHcy. Furthermore, dietary amounts of folate and vitamins B6 and B12 are inversely related to plasma tHcy levels. Plasma tHcy levels are on average 25 percent higher in men than in premenopausal women, which may be related to gender differences in the efficiencies of the metabolic pathways.
| Causes of Elevated Plasma Homocysteine |
| Genetic Factors |
| Methylenetetrahydrofolate reductase Cystathionine ß-synthase Methionine synthase |
| Nutritional factors |
| Folate deficiency Vitamin B6 deficiency Vitamin B12 deficiency Excessive methione intake |
| Demographic factors |
| Decreased renal function Diabetes mellitus Hypothyroidism Severe psoriasis Malignant neoplasia Organ transplantation |
| Drugs |
| Alcohol Tobacco Caffeine Nitrous oxide Anticonvulsants Methotrexate Cyclosporine A |
| Chronic diseases |
| Decreased renal function Diabetes mellitus Hypothyroidism Severe psoriasis Malignant neoplasia Organ transplantation |
Homocysteine and Atherosclerosis
A link between homocysteine and arteriosclerosis was first hypothesized after marked atherosclerotic-like lesions were observed in two neonates with homocystinuria. The exact mechanism by which homocysteine causes atherogenesis is unclear, but it may be mediated through generation of reactive oxygen species such as superoxide anions and hydrogen peroxide that can cause direct endothelial cell damage and reduction in nitric oxide levels.
Other prothrombotic effects of homocysteine include activation of factors V, X and XII, and direct or indirect inhibition of protein C, thrombomodulin, heparin sulfate and tissue plasminogen activator. Homocysteine also promotes development of atheroma and vascular thrombosis by inducing smooth muscle proliferation, lipid peroxidation, oxidation of low-density lipoproteins and elevation of triglyceride levels.
Epidemiologically, the role of elevated plasma tHcy levels in the development of cardiovascular disease is controversial. Some argue that plasma tHcy is a result, not a cause, of systemic vascular occlusive disease or that it is an epiphenomenon secondary to subclinical renal atherosclerotic disease and reduced renal clearance of plasma tHcy. Early, retrospective reports showed that elevated plasma tHcy is an independent risk factor for venous thrombosis, and coronary, cerebral and peripheral vascular occlusive disease. Further, plasma tHcy interacts with other risk factors such as hypertension and hypercholesterolemia.
Several prospective studies have provided further evidence supporting the role of plasma tHcy in systemic vascular occlusive disease. These concluded that elevated plasma tHcy was a modest to strong predictor of ischemic heart disease and stroke in healthy populations and that a reduction of plasma tHcy by 3 µmol/L would significantly reduce the risk of ischemic heart disease, deep vein thrombosis and stroke.
Similarly, a prospective study revealed that plasma tHcy levels were strong predictors of ischemic stroke among patients at increased risk due to chronic ischemic heart disease. Interestingly, two studies demonstrated that individuals with the TT genotype had an increased risk of ischemic heart disease or stroke, but this was related to the effect of low folate levels in these persons rather than the genetic mutation.
Homocysteine and Diet
As previously mentioned, dietary levels of the pivotal compounds folate vitamin B6 and vitamin B12 are inversely related to plasma tHcy levels. Eating more B vitamins, particularly B12, and folate can lower plasma tHcy levels by 15 percent and raise serum folate levels as evidenced by feeding studies.
Alternatively, daily folate and vitamin B12 supplements of 500 µg or less can reduce plasma tHcy levels by up to 30 percent, with the greatest reductions seen in persons with the highest pre-treatment plasma tHcy levels. Vitamin B6, primarily a co-factor in the transsulfuration pathway, may not be so effective in lowering tHcy, and its role in lowering plasma tHcy levels needs to be further studied. All three of these supplements are inexpensive and available over the counter, although combined folate B12 supplementation is prudent in order to avoid the theoretical risk of neuropathy secondary to unopposed folate therapy in B12-deficient patients.
Randomized, placebo-controlled trials examining the effect of folate and B- vitamin supplementation on cardiovascular disease are currently under way worldwide and may confirm a link between major cardiovascular events and mildly elevated plasma tHcy levels.
Retinal Vascular Occlusive Disease
We recently performed a meta-analysis of published data on the relationship between plasma tHcy, serum folate, serum vitamin B12, TT genotype, and retinal vascular occlusive disease. In total, 614 patients in nine reports with all types of retinal vein occlusion had significantly higher mean plasma tHcy levels than 762 controls. Analysis by retinal vein occlusion type showed that 465 patients from seven studies had significantly higher mean plasma tHcy levels than 658 persons without the disease.
Similarly, 129 patients in three studies of BRVO had significantly higher mean plasma tHcy levels than 256 controls. Four reports on elevated plasma tHcy in patients with retinal artery occlusion showed that mean plasma tHcy levels were significantly higher in 154 patients than 358 controls.
Only four studies examined the relationship between plasma tHcy, its dietary determinants and retinal vascular occlusion. Mean serum folate levels were significantly lower in 287 patients with any type of retinal vascular occlusion compared with the same number of controls. However, no difference appeared in the mean serum vitamin B12 levels in either the cases or controls in the same four studies. There was no data on the relationship between vitamin B6 levels and retinal vascular occlusive disease.
Nine studies examined TT genotype and retinal vein occlusion. The prevalence of the TT genotype was similar in 690 patients with all types of retinal vein occlusion compared with 2,754 controls. Likewise, analysis of three studies of retinal artery occlusion demonstrated no significant difference in the proportions of patients (n=152) and controls (n=435) with the TT genotype.
However, there was a trend towards the TT genotype in patients with retinal vascular occlusions, particularly in Mediterranean patients. Ethnic or geographic variations may explain the difference in the prevalences in the TT genotype in some studies included in the meta-analysis. While MTHFR is a pivotal enzyme in the remethylation pathway of homocysteine metabolism, the relationship between TT genotype and higher plasma tHcy levels is unclear. This relationship is likely strongly affected by a person's dietary intake of folate; and it appears that serum folate levels, rather than TT genotype, are the true risk factor for retinal vascular occlusion.
Estimation of plasma tHcy and serum folate levels should be considered in patients with retinal vascular occlusion as correction of these risk factors may reduce the risk of another vascular occlusion in the fellow eye. Identification of elevated plasma tHcy and low serum folate levels may also reduce the patient's risk of developing a cardiovascular event. At present, measurement of serum vitamin B6 and B12 levels may not be necessary, as there is no published data on the role of vitamin B6 in retinal vascular occlusions, and serum vitamin B12 levels were not significantly associated with the disease. Similarly, determination of MTHFR genotype is not recommended at present in evaluating patients with retinal vascular occlusions. Folate supplementation at a dose of at least 400 µg/day should be considered in patients with retinal vascular occlusion who have elevated plasma tHcy and low serum folate levels.
The addition of vitamin B12 at an oral dose of 400 µg/day or more protects against the development of subacute combined degeneration of the spinal cord, but as vitamin B6 has not been shown to reduce plasma tHcy levels, supplementation with this vitamin should be at the physician's discretion. Ongoing prospective studies on the effects of folate and B vitamin supplements on lowering plasma tHcy levels may result in modification of these recommendations.
Dr. Fekrat is an associate professor of ophthalmology at the Duke University Eye Center. She specializes in the medical and surgical treatment of vitreoretinal diseases. Contact her at Duke University Eye Center, Erwin Rd., PO Box 3802, Durham, NC 27710. Phone: (919) 681 0341; fax: (919) 681 6474; e-mail: fekra001@mc.duke.edu. Dr. Cahill is is a clinical associate in the Vitreoretinal Service at Duke.
1. Brown GC. Arterial occlusive disease. In: Regillo CD, Brown GC, Flynn HW, editors. Vitreo-retinal disease: the essentials. New York: Thieme, 1999:97-115.
2. Fekrat S, Finkelstein D. Venous occlusive disease. In: Regillo CD, Brown GC, Flynn HW, editors. Vitreo-retinal disease: the essentials. New York: Thieme, 1999:117-32.
3. Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 1998;338:1042-50.
4. Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B12 and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol in press.