Although new surgical approaches for treating glaucoma continue to appear, drugs remain the first-line therapy for most doctors. Currently, several companies and university departments are researching highly promising new alternatives.
Most new drugs that could reach the marketplace within the next year or two are existing drugs with preservatives removed or altered, or combinations of familiar drugs. But other less familiar approaches are also under investigation. Two of the most promising are neuroprotection—compounds that prevent cell damage even under high intraocular pressure—and drugs that act directly on the trabecular meshwork to increase aqueous outflow.
Companies investigating new options are usually reluctant to discuss the status of their discoveries. (Allergan's memantine, currently in Phase III trials, is a case in point.) However, some surgeons and researchers were happy to discuss their work with us, giving us a glimpse into what may lie ahead.
Focus: the Trabecular Meshwork
David L. Epstein, MD, chairman of the Department of Ophthalmology at Duke University School of Medicine in
Today, Dr. Epstein is aware of at least five groups pursuing new ways of altering the trabecular meshwork, and he sees this as perhaps the most logical approach to treating glaucoma. "Currently, I think we treat glaucoma nonspecifically," he says. "Abnormalities in pressure are involved in at least 90 percent of the glaucomas, and pressure goes up because of abnormal drainage through the trabecular meshwork. Yet we have no drugs targeted at the TM. Of course, we have aqueous suppressants such as the beta-blockers and alpha-2 agonists, and prostaglandins which very effectively divert flow through the uveoscleral outflow pathway, possibly with a small effect on the TM.
"However," he continues, "with time, people treated with these drugs still tend to get worse. It's not that the prostaglandins or beta-blockers lose their effectiveness; it's that in glaucoma—and normal aging, for that matter—outflow through the TM gradually gets worse. And as the drainage problem gets worse, the pressure creeps back up. We try to stay ahead of it by adding another drug, but we're not addressing the real problem. To me, that's the definition of palliation."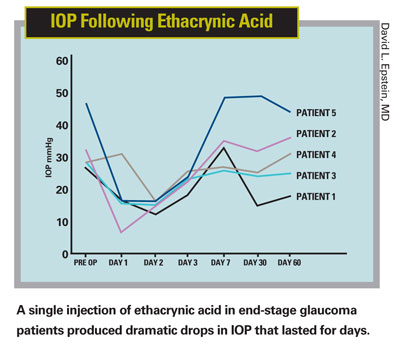
Dr. Epstein says he doesn't mean to minimize the importance of neuroprotection. "Clearly, a part of glaucoma involves abnormality in the ganglion cells," he says. "Preventing this from allowing or causing cell death is a very worthwhile effort. But studying this part of the problem is difficult, in part, because elevated eye pressure is a confounding factor. If we could normalize outflow, eye pressure wouldn't fluctuate much; researchers could really focus on what's happening in the back of the eye and develop drugs for patients who have very susceptible optic nerves or ganglion cells."
The Ethacrynic Acid Connection
Dr. Epstein says his interest in ethacrynic acid resulted from his desire to develop a drug that's specific for the outflow pathway—a drug that would not only normalize outflow, but would reverse abnormal function and perhaps cure the pressure component of glaucoma. "Ethacrynic acid is a good starting point because it's a cytoskeleton-active drug," he explains. "Every cell has a cytoskeleton, a scaffold of proteins that gives the cell its shape and anchors its attachment to other cells. Ethacrynic acid alters the cytoskeleton and causes the shape and attachments of the cell to change."
Dr. Epstein notes that ethacrynic acid has a poor reputation because of a clinical trial that failed to achieve its desired endpoints, leading many people to conclude that this compound was totally ineffective. "The idea behind that trial was to use ethacrynic acid to treat the temporary glaucoma that occurs after cataract surgery, when the TM appears to be plugged up by viscoelastic remnants," he explains. "The problem with the trial, discovered after the fact, is that ethacrynic acid binds to viscoelastic. The compound was administered at the end of each case; as a result it was binding to remnants of the viscoelastic and not even getting into the TM. Of course, even if this protocol had worked, the temporary rise in pressure that can occur after cataract surgery is not the same as primary open-angle glaucoma.
"In contrast," he says, "Dr. Shlomo Melamed at the Goldschleger Eye Institute in

Getting into the Eye
Dr. Epstein notes that one of the advantages of ethacrynic acid is that it's an approved systemic drug. "It's often used as a diuretic," he says. "It's being injected into human veins and doesn't cause side effects (other than being a diuretic).
"The problem with ethacrynic acid, and second- and third-generation compounds that have been developed since, is that they don't penetrate the cornea very readily," he continues. "What we started to do at Duke University, before we licensed the idea to AERIE, was to form analogues of ethacrynic acid that could penetrate into the eye better, as well as finding alternate ways to deliver the drugs besides eye drops.
"Injecting a drug into the eye has become more acceptable recently, thanks to the advent of anti-VEGF drugs that are injected to treat age-related macular degeneration, so that's one option," he notes. "Of course, ethacrynic acid has already been injected into the anterior chamber of humans with no negative effect on the cornea or endothelium. However, the drug has to be long-lasting enough to minimize the number of injections needed."
Dr. Epstein says that, ideally, they'd like to find a noninvasive way to administer a compound of this type. "If you're having trouble getting a drug into the eye, one approach is to develop a drug that's a thousandfold more potent; then it will work even if only a tiny bit gets inside the eye," he says. "You can also try to figure out why it doesn't get through the cornea and modify it so it does get through. An implant could work as well, if it lasted long enough. All these options are being considered.
"The bottom line is that I know unequivocally that if we can get the drug to the TM, it's going to work," he concludes. "We just need a way to make sure the drug gets to the target in a high enough concentration."
Anecortave Acetate
Alan L. Robin, MD, clinical professor of ophthalmology at the
Dr. Robin describes how his first use of the drug to treat ocular hypertension came about. "In 2000, a patient was sent to me by a retina specialist, Dr. Eric P. Suan, who knew I was aware of the potential of anecortave acetate," he says. "The patient was a 35-year-old diabetic who had lost one eye to advanced proliferative diabetic retinopathy. The second eye had advanced neovascular glaucoma with blood vessels everywhere in both the anterior and posterior segment after total panretinal photocoagulation. The patient was on maximal therapy and still had a pressure of 45 or 50 mmHg in his good eye.
"We were desperate to save the good eye, so we injected 0.8 ccs of anecortave acetate as an anterior juxtascleral depot," he continues. "The results were remarkable: The smaller blood vessels started going away within a month. Later, the big vessels disappeared, and finally we couldn't detect any abnormal anterior segment vessels. Correspondingly, his IOP went down and stayed down—for more than six years! I saw him most recently about a month ago. His diabetes has progressed, as he is non-compliant with his therapy, and his anterior segment neovascularization has finally reappeared. However, his IOP is still less than 21 mmHg on no IOP-lowering medications."
Dr. Robin says his second encounter came several years later. "A patient was sent to me who had a marked pressure rise caused by an intravitreal steroid injection," he recounts. "Usually that kind of pressure rise lasts about six months, and I knew that the effect of anecortave acetate supposedly lasted about six months. So I delivered an anterior juxtascleral depot of anecortave acetate in the sub-Tenon's, and the patient's pressure went down.
"The next question was, would this work with open-angle glaucoma?" he continues. "We tried it in six or seven eyes with a moderate degree of primary open-angle glaucoma, with the FDA allowing our protocol. Five of the seven eyes had remarkable pressure lowering that lasted for months. One patient is currently still doing well after a year.
"The seven-year pressure lowering I first encountered is probably an exception," admits Dr. Robin. "We really don't know. But I've tried this on about 30 patients so far, and the shortest the effect has lasted is almost six months from a single injection. This agrees with the data from David Callanan, a retina specialist in
Practical Considerations
Though injections are still a point of concern for many patients and ophthalmologists, Dr. Robin says this part of the procedure is not overwhelming. "The injection is comfortable for the patient; you just put some topical proparacaine on the eye," he says. "So far it seems to be very safe and easily tolerated. It's not intravitreal, so there's no risk of endophthalmitis. Most patients are initially apprehensive about having the injection, but afterwards they're very happy because they hate taking eye drops."
As far as how the drug behaves in the eye, Dr. Robin says anecortave acetate is very insoluble. "It stays around for a long time and generally doesn't diffuse very well," he explains. "That's why it was used for retina diseases. However, if you inject the drug into the anterior chamber the way I've been doing it, it migrates to cover the trabecular meshwork, almost 360 degrees. Of course, that's where you'd want an outflow drug to be to maximize its effect. It doesn't block the aqueous; it doesn't go into Schlemm's canal or into the trabecular meshwork. It just slowly dissolves and somehow enhances outflow."
Dr. Robin says Alcon is currently conducting multicenter FDA Phase II studies evaluating the IOP-lowering efficacy of anecortave acetate in open-angle glaucoma. He adds that Jorge A. Alvarado, MD at UCSF in
Dr. Robin believes a drug like this could cause a major paradigm shift in the treatment of glaucoma. "Patient compliance is a huge issue—both in terms of whether the patient actually remembers to use the eye drop, and whether he really gets it onto his eye," he notes. "A treatment that lasts six months would eliminate all of those concerns. Almost all glaucoma patients see their ophthalmologist at least twice a year. With a treatment like this, as long as you see your doctor, you're pretty much okay.
"This drug clearly has a lot of potential," he concludes. "Hopefully we'll soon know what its real potential is."
The Neuroprotection Factor
Arthur H. Neufeld, PhD, professor of ophthalmology at Northwestern University School of Medicine in
"That was an initial finding," he explains. "We had previously demonstrated that inducible nitric-oxide synthase (iNOS) was associated with glaucoma, and that NO made by iNOS could be destructive to the axons in glaucoma. In the 1999 study we used aminoguanidine, an iNOS inhibitor, to minimize the ability of the enzyme to make NO, on the theory that it would be protective. In our rat glaucoma model, that was in fact what we found.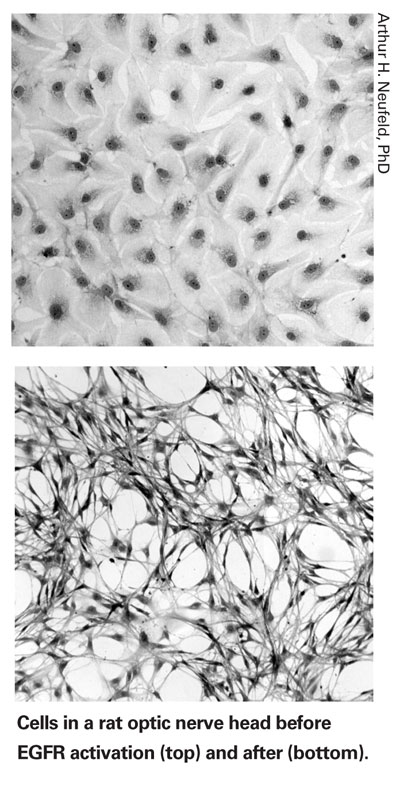
"Since then," he continues, "we've asked the question: Why is iNOS appearing in glaucomatous tissue? What's stimulating its appearance? To answer that question, we 'went upstream' looking for the signal that causes the cell to make this destructive enzyme. The idea was to try to find the precursor so we could stop the entire process from occurring, and thus prevent the offending enzyme from ever appearing."
Treating the Source
"Over the past four or five years we've been following this trail, searching for the initial signal that triggers synthesis of this destructive enzyme," he says. "We finally did identify it: The initial signal is the Epidermal Growth Factor Receptor in astrocytes.
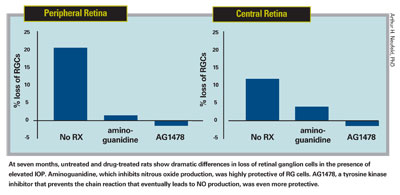
"When you stimulate that receptor, many things happen to the astrocyte, including the synthesis of iNOS," he continues. "So, to see whether blocking this chain of events would prevent retinal ganglion cell destruction, we used an inhibitor of EGFR in a rat glaucoma model. We found that it completely inhibited loss of retinal ganglion cells in the presence of elevated IOP—even more effectively than aminoguanidine. [See graphs, below.] The EGFR inhibitor prevented the gene from ever being expressed, so the iNOS never appeared." (A report on this study was published in 2006.2)
"What we showed in the recent article is that the EGFR pathway changes the astrocytes from quiescent, normal astrocytes into reactive astrocytes," he explains. "Based on what we've seen, we think that EGFR is the key factor for turning an astrocyte into a destructive element in the eye or brain—it activates a significant number of changes that should not occur in normal tissue, triggering all kinds of abnormal cell responses. In fact, this can occur in many types of neurodegenerations, not just glaucoma."
At seven months, untreated and drug-treated rats show dramatic differences in loss of retinal ganglion cells in the presence of elevated IOP. Aminoguanidine, which inhibits nitrous oxide production, was highly protective of RG cells. AG1478, a tyrosine kinase inhibitor that prevents the chain reaction that eventually leads to NO production, was even more protective.
Proving It Works
As it happens, two EGFR inhibitors, Tarceva (erlotinib, OSI Pharmaceuticals) and Iressa (gefitinib, AstraZeneca), are already on the market; they are currently being used clinically to inhibit the growth of cancer cells in tumors. Dr. Neufeld says he and his fellow researchers have discussed the possibility of using these drugs as a neuroprotective glaucoma treatment with the manufacturers extensively, but they're not interested.
"These drugs are already being used in people, but the companies are not willing to conduct a glaucoma-related clinical trial," he says. "They don't have any current ophthalmic products, and a trial would be expensive. Also, the clinical endpoint is difficult when you're talking about trying to prevent the degeneration of the optic nerve. It's not like simply measuring IOP. So, for business reasons more than scientific reasons, I haven't been able to get the companies to go for it.
"Any glaucoma doctor could do a small-scale study," he notes. "You could put 30 patients on Tarceva and follow them for a few years and see what happens. But it's a difficult study to do. You have to know what the normal progression is going to look like and see if you can change the slope of that progression. And it still won't be as definitive as a large clinical trial with hundreds of patients."
In terms of application, Dr. Neufeld notes that these compounds don't need to be given by intravitreal injection. "In our animal studies we gave the drug by mouth," he says. "We put it in their drinking water. We were able to show that the drug got to the eye and worked on the optic nerve.
"Can the same thing be done in people?" he asks. "I don't know. But I think the first attempt in people using the existing cancer drugs would be oral, just the way they give it for cancer, and the way memantine is being tried by Allergan as a neuroprotective agent."
As to whether this would entail side effects, Dr. Neufeld says that information is known primarily to the companies manufacturing the drugs. "Clearly, these drugs are not going to kill the patient," he says, "but there's no public knowledge of side-effect profiles based on data from large trials. That's another reason you would need to have the company involved in any clinical trial."
Despite his frustration about being unable to convince the current drug manufacturers to sponsor a clinical trial, Dr. Neufeld believes this class of drug has immense potential as a weapon against the ravages of glaucoma. "If I had glaucoma," he says, "I'd want to be treated with this."
1. Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci
2. Liu B, Chen H, Johns TG, Neufeld AH. Epidermal Growth Factor Receptor Activation: An Upstream Signal for Transition of Quiescent Astrocytes into Reactive Astrocytes after Neural Injury. 2006; 26:28:7532–7540.