Presbyopia Treatment
First Presbyopia Drop Approved
Allergan/AbbVie announced FDA approval of Vuity (pilocarpine HCl ophthalmic solution) 1.25% for the treatment of presbyopia in adults, as the first FDA-approved drop to treat this condition. The company says that Vuity is a daily prescription drop that works as soon as 15 minutes after use and lasts up to six hours, as measured on day 30, “to improve near and intermediate vision without impacting distance vision.” Vuity is a formulation of pilocarpine delivered with “pHast” technology, enabling the drop to rapidly adjust to the physiologic pH of the tear film, Allergan says.
The approval was based on data from two pivotal Phase III clinical studies, GEMINI 1 and GEMINI 2. In both studies, Allergan says, Vuity met the primary endpoint, reaching statistical significance in improvement in near vision in mesopic conditions without a loss of distance vision, versus the vehicle (placebo), on day 30 at hour three. Additionally, improvement was seen as early as 15 minutes and lasted through six hours. There were no serious adverse events observed in participants receiving Vuity in either the GEMINI 1 or GEMINI 2 study. The most common adverse events, occurring in less than 5 percent of patients, were headache and eye redness, the company says.
For more information, visit vuitypro.com.
Ocular Surface
Dextenza Gets New Indication
Ocular Therapeutix announced that the FDA has approved its Supplemental New Drug Application to broaden the Dextenza label to add an additional indication for the treatment of ocular itching associated with allergic conjunctivitis. The intracanalicular insert lasts for up to 30 days, the company says. Dextenza is already approved to treat ocular pain and inflammation following ophthalmic surgery.
For information, visit bausch.com.
Contacts
Menicon lens
Menicon has released its first silicone hydrogel daily disposable contact lens. The company says the Miru 1day UpSide lens provides high oxygen availability and has an ultra-low modulus and a smooth, low-friction surface to promote eye health. The packaging ensures that the lens is always sitting convex-side up on a small bump to make handling the lens easier for patients, as well as minimizing risk of inner-lens contamination.
For information, visit meniconamerica.com.
Testing and Planning
Help for Detecting Oculomotor Dysfunction
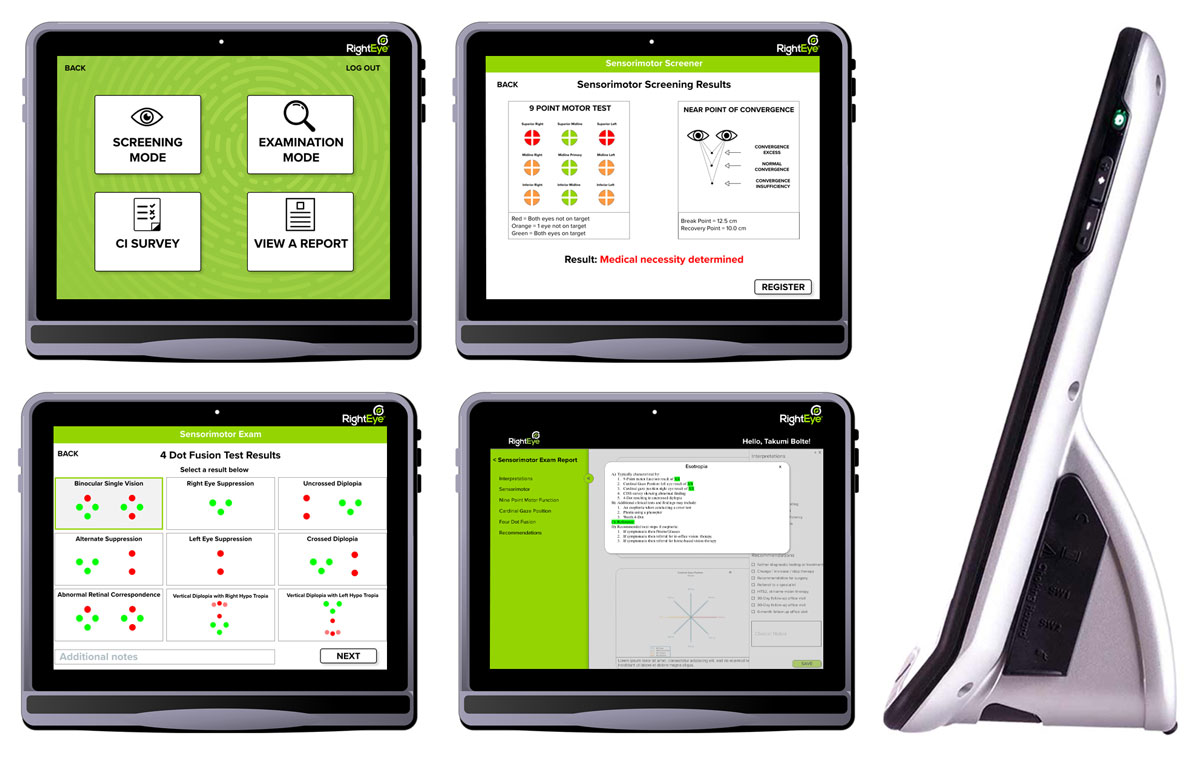 |
If you want to improve the accuracy of your EOM exams—and reduce the time it takes to perform them—a new product from RightEye aims to help, the company says. Called RightEye Sensorimotor, the device is a tablet with an eye tracker and custom software that administers oculomotor tests and then produces documentation of the results.
Because the test can be run with minimal input from an operator, doctors are able to remove this element from their exams and delegate it to a technician in the pretest area, the company suggests.
The data generated can give clinicians better documentation of a patient’s status upon identification of a problem, which can then be used as a baseline for comparison in follow-up visits, RightEye says.
For more information, visit RightEye.com.
Alcon Introduces Smart Cataract Software
Alcon recently released its Smart Solutions platform, starting with its Smart Cataract System. Using this software, surgeons and their staff can enter data once and have it carried forward throughout the office, from EHR systems to diagnostic equipment and surgical devices. The application connects data systems and most diagnostic devices—including the Argos Biometer with Image Guidance—with Alcon’s cataract surgical equipment, including the LenSx Femtosecond Laser and LuxOR Revalia Ophthalmic Microscope, as well as commonly used microscopes and devices from other ophthalmic manufacturers. The company says that connecting biometry instruments with other medical records expedites surgical planning. Alcon says entering the data only once will also help eliminate transcription errors.
SMART Solutions makes use of the open, cloud-based infrastructure and services of Philips HealthSuite, built on Amazon Web Services, to enable surgeons to aggregate, organize and analyze all relevant data within a single, integrated application “designed for security and remote planning.”
Alcon is installing the system in several additional practices in the United States through the end of the year, and plans a broader rollout of Smart Cataract in 2022.
To learn more, visit AlconSMARTSolutions.com.