The race to find a treatment for presbyopia—or at least its symptoms—continues unabated. Monovision, in which one eye is given a distance focus and the fellow eye a slightly nearer focus, has been a popular way to address this for many years. That’s been true in part because the alternatives (multifocal intraocular lenses, for example) can have drawbacks such as glare and haloes that not all patients are willing to tolerate, in addition to being considerably more expensive. But monovision also has drawbacks, notably the loss of summation between the two eyes, limiting how much difference can be created. Nevertheless, several of the newer options profiled below take advantage of monovision to extend the vision range their approach provides.
Here, we’ll review 11 options that are attempting to treat the limited vision caused by presbyopia without resorting to implanting a multifocal IOL. They can be thought of as falling into three categories: 1) pharmaceuticals that use miosis to take advantage of the pinhole effect; 2) surgical approaches; and 3) pharmaceutically altering the crystalline lens or cornea.
Pharmaceutical Miosis
One of the most intriguing avenues of research into presbyopia correction involves the use of topical drops to alter the pupil in order to confer near vision. Here’s the latest:
• The Orasis Drop. An eye drop designed to relieve symptoms of presbyopia using miosis is being developed at Orasis, a company based in Israel. Asked what makes the Orasis drop innovative, CEO Elad Kedar says that one of the keys is finding the miosis “sweet spot.” “You need to constrict the pupil just the right amount,” he explains. “If you constrict the pupil too much, it can have a negative impact on distance vision, night vision and visual field, among other things. So the key is to find the amount of miosis that will give the person great visual acuity without causing any negative phenomena. And of course, the drop has to avoid causing adverse events such as headache, brow ache and red eye. This is especially important in a quality-of-life drug; there can be no compromise on safety and tolerability in this situation.
“The three key elements we see as essential are efficacy, safety and comfort,” he continues. “We want people to be able to use our drug on a daily basis. We want the drop to be comfortable when they administer it, we want them to have freedom to choose when to use it, and finally, we want the drop to start working fast and last a good length of time. So far, the data from our studies show that we’ve reached that optimal balance. Furthermore, this is intended for use in both eyes.”
 |
Mr. Kedar notes that the active ingredient in the drop is low-dose pilocarpine. “We’re using a concentration significantly lower than that used with glaucoma,” he says. “Also, our drug has a multifaceted vehicle that works with the pilocarpine to create that optimal balance of efficacy, safety and comfort. The onset of action is rapid; studies so far show significant results after 20 minutes. In terms of duration, the effects are still significant after several hours. For some people, especially younger people, it could last even longer.
“We’re aware of what competitors are working on, and our drop is definitely different from theirs in a number of respects,” he adds.
Asked about downsides to the drop, Mr. Kedar says they’ve worked hard to ensure there wouldn’t be any. “This is a temporary, noninvasive way to address presbyopia,” he points out. “Some people will use it every day, others will only use it occasionally. We’re not trying to eliminate reading glasses. We’re trying to provide freedom of choice and a great temporary solution that doctors can offer their patients, benefiting both parties.”
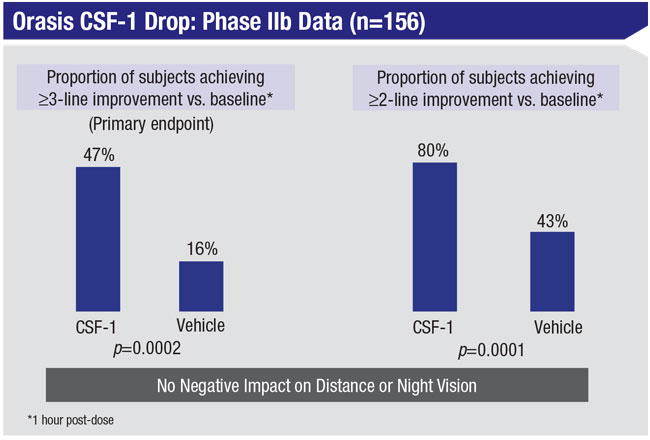
Mr. Kedar says the company completed a Phase IIb multicenter, double-masked clinical trial involving 166 participants in 2019. “We met the primary endpoint successfully,” he notes. “Participants gained at least three lines of improvement at near and the data showed no reduction in distance vision or night/low-light vision, which is a key element of efficacy. In fact, the data showed a trend toward improved distance vision. All of the safety and tolerability endpoints were met, as well. That’s allowing us to go directly into a Phase III trial, which should be starting soon.”
Mr. Kedar adds that they recently completed Series C funding that raised $30 million to advance Orasis’ lead eye drop candidate through completion of its Phase III clinical trials, and for pre-commercialization activities ahead of a potential product launch.
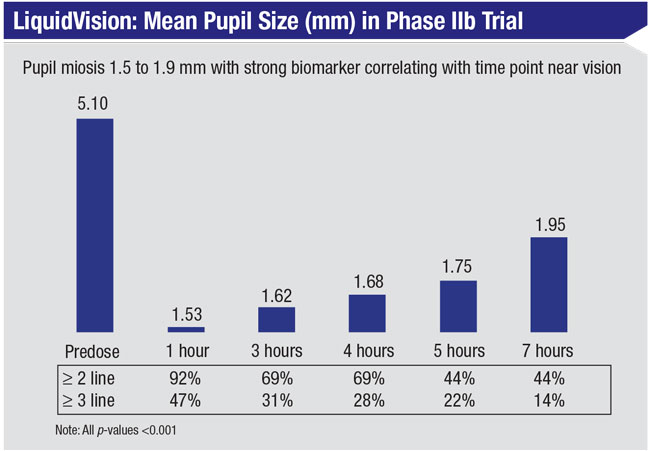
• Liquid Vision. Presbyopia Therapies, a company based in Coronado, California, has developed a drop called LiquidVision that creates miosis without inducing myopia, to address the symptoms of presbyopia. Unlike some other presbyopia drops that use pilocarpine, LiquidVision’s active ingredient is aceclidine, which has a different mechanism of action that the company says produces a greater depth of field than pilocarpine. Data from the company-sponsored Phase IIb study showed that this drug produced a pupil size ranging from 1.5 mm to 2 mm, without inducing any blurring at distance. The company says the drop allows patients to see both near and far simultaneously, from 16 inches to infinity.
Jim McCollum, CEO of Presbyopia Therapies, explains that aceclidine’s MOA should make it possible to treat the symptoms of presbyopia in a wider age range and broader refractive error range than pilocarpine. “LiquidVision can treat symptoms in people from ages 45 to 70, with between -4.5 and +1.5 D of spherical error and up to
2 D of astigmatism,” he says. “That’s 80 percent of the bell-shaped curve. Pilocarpine-based drugs may work fine, but they’ll work for a much narrower group of patients and will likely be more of a niche product.
“Both pilocarpine and aceclidine have been in use for 40 or 50 years, so they have massive amounts of safety data,” he continues. “Aceclidine, by its nature, is temporary and reversible. In the Phase IIb study, some patients saw well for eight hours with one dose. Some patients may use it once a day, but the FDA label will most likely be for b.i.d. dosing.”
Mr. McCollum notes that the data hasn’t revealed any significant change in night vision/low-luminance conditions. “Good night vision is an important regulatory and commercial endpoint,” he says. “Some patients may choose to use the drug at night because it filters out glare and haloes, and the data show no loss of vision at night. If you’re trying to read a menu in a candlelit restaurant, you might be challenged, but if you’re just functioning normally, for example, driving at night, you shouldn’t have issues. Incidentally, when you sleep at night, the drug washes out, and by morning, you’re back to your original baseline. We consider that a safety component of the drug.”
Mr. McCollum says the drop might be contraindicated in patients who have had some types of retinal surgery or have an early cataract, which would limit its efficacy. “We expect that interested patients could sample it in a doctor’s office,” he says. “It only takes 20 to 25 minutes to act, so you can tell very quickly if a given patient is getting a good response.”
Mr. McCollum says the Phase II trial has been completed, with a Phase III trial expected to begin in 2021.
 |
• Allergan/Abbvie’s AGN 190-584 Drop. Another entry in the competition to create an ideal topical presbyopia drop is Allergan’s AGN 190-584. “AGN-190584 is an experimental formulation of pilocarpine, a cholinergic muscarinic receptor agonist, that’s being investigated for presbyopic near vision correction,” explains Michael R. Robinson, MD, vice president and global therapeutic area head of ophthalmology at Allergan. “This will be a topical, once-daily drop delivered by a proprietary vehicle.”
Initial studies tested pilocarpine in tandem with oxymetazoline, a direct-acting alpha-1 adrenergic agonist and alpha-2a adrenergic partial agonist. Data from the company’s Phase IIb study involving 151 individuals, meant to determine the efficacy of different formulations of the drop, were recently presented at the annual American Academy of Optometry meeting.
Subjects were divided into five groups; one served as the control, while the others received different combinations and strengths of the two key drugs. All participants received one drop per day for 28 days. Results included:
• Improvements in vision were seen within 15 minutes in all groups; peak improvement was seen at one hour. Vision continued to improve from day one to day 21, and was maintained at day 28.
• In the groups receiving stronger doses, gain in mesopic UNVA at day 28 ranged from 7.54 to 7.81 letters.
• All treatment groups demonstrated efficacy compared to vehicle, and there was no sign of tachyphylaxis during the 28-day dosing period.
• Headache was the most common adverse event, reported by 16.7 percent to 28.1 percent of the different treatment groups. (Notably, 10.7 percent of the control group members also reported experiencing headache.)
Phase III studies of the latest formulation—GEMINI I and GEMINI II—are currently ongoing.
• Brimochol. Another potential treatment for the symptoms of presbyopia is under investigation by Visus Therapeutics (Irvine, California). Their drop combines two active ingredients: carbachol and brimonidine tartrate. According to the company, five clinical studies have already been conducted. The most recent study involved 57 patients, and showed a near-vision improvement of five lines or more (a statistically significant difference) that lasted at least 12 hours. Participants reported no headaches or brow aches.
The company recently formed a clinical advisory board headed by Eric Donnenfeld, MD, joined by a number of high-profile ophthalmologists including Ed Holland, MD, Marguerite
McDonald, MD, FACS, William
Trattler, MD, and George Waring IV, MD, FACS.
Phase II trials are expected to begin in 2021.
• FOV Tears. An eye drop developed by Colombian ophthalmologist Luis Felipe Vejarano, MD, is showing promise as a treatment for the symptoms of presbyopia. The drop is intended to create what Dr. Vejarano calls “dynamic pseudoaccommodation,” which combines a small amount of miosis with improved accommodation. Early studies showed that the drop doesn’t impact distance vision.
The drop, which contains pilocarpine and several other components, can be used bilaterally. Onset of action and duration of effect apparently improve with long term use; onset takes five to 10 minutes for most users by the third month of use. The effect lasts four to five hours at first, but may last up to eight hours after extended use. Most patients have been using the drops two times a day, once in the morning and once around mid-afternoon. Some patients also use a third drop in the evening, depending on their vision needs.
Several studies have been published, the most recent in 2019.1 In that prospective, interventional, non-comparative study, 117 presbyopic patients were given one drop in each eye. Patients were divided into two groups: age 41 to 50 years old, and age 51 to 65 years old. Two hours after instillation of the drops their binocular uncorrected near visual acuity (UNVA) and uncorrected distance visual acuity were evaluated. Researchers also assessed subjects’ objective scatter index and pupil diameter under photopic and scotopic conditions, before and after instillation.
Findings included:
• The mean UNVA before instillation was 0.35 logMAR (20/45). At two hours after instillation, logMAR improved to 0.16 (20/29).
• Near vision improved by at least one line in 92.3 percent of the patients at two hours.
• Nine patients (7.6 percent) failed to show an improvement.
• No patients lost any lines.
• Fourteen patients (11.9 percent) reported experiencing headaches.
• The younger age group gained more lines than the older group.
Jorge L. Alió, MD, PhD, a professor and Chairman of Ophthalmology at the University Miguel Hernandez de Elche, in Alicante, Spain, has conducted several clinical trials using the drops. (He has no financial ties to the drops.) “Our first pilot study showed that the drop made a significant difference in patients with early or intermediate presbyopia,” Dr. Alió explains. “It even worked in presbyopes with previous refractive surgery, whether intraocular or cornea-based, including patients who had undergone presbyLASIK.”
Dr. Alió notes that FOV Tears don’t work equally well in all presbyopes. “In our studies, about 70 percent of the patients gave a positive evaluation of the medication. However, about 30 percent were either not improved—possibly because their presbyopia was too advanced—or they had some minor side effects that discouraged them from using the medication,” he says. “In my experience, this formula is effective in all patients with early or intermediate presbyopia, eliminating the need for reading glasses. Once we know which are the best cases to treat, I think this will be successful in more than 90 percent of patients.
“My wife has been using these drops for almost three years,” he adds. “She’s happy and doesn’t need any other solution for her presbyopia.”
FOV Tears have been approved for use in Spain, but are not currently available in the United States.
Surgical Approaches
Here’s an update on more invasive methods for treating presbyopia, some of which have been in the works for a while:
• KAMRA. Several corneal inlays have been developed over the years, hoping to improve the range of vision in presbyopes, but only one is currently available: the KAMRA inlay (initially brought to the marketplace by AcuFocus, now available from SightLife Surgical/CorneaGen).
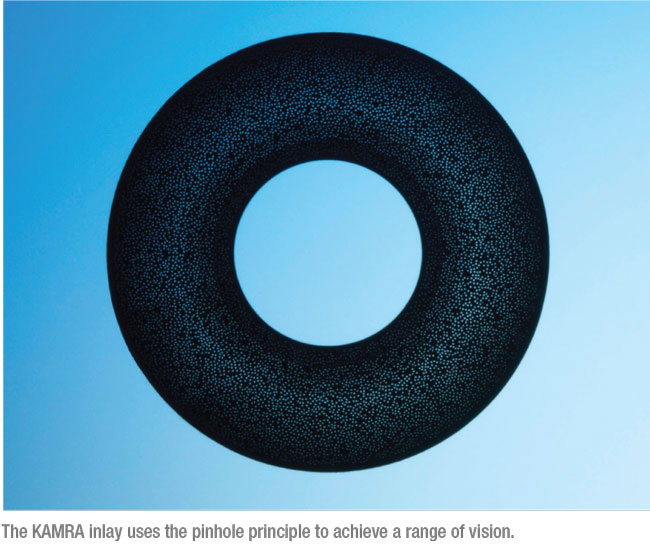 |
The 5 µm thick, 3.8-mm diameter polyvinylidene fluoride device features a 1.6-mm central aperture; it uses the pinhole principle to improve range of vision. The device has more than 8,000 microperforations of different sizes designed to allow oxygen and nutrients to pass through. It’s implanted in a 200-µm deep femtosecond-laser-created pocket in the cornea of the non-dominant eye. To work effectively, vision should be between -1 and plano before the surgery, which can be accomplished via LASIK in a separate procedure, if necessary.
Although surgeons agree that the device is effective, the KAMRA inlay hasn’t swept the presbyopia market. That may be because A) most people don’t know someone else who’s already had one implanted; B) it requires adjusting to some degree of monovision, since it’s meant to be implanted only in one eye; C) it may require an additional procedure to get the patient to emmetropia before implantation; and D) it’s not inexpensive. Nevertheless, it a valuable option to offer patients—and unlike many presbyopia options in development, it’s available now.
• The PEARL Procedure. In addition to pharmaceutical options, doctors are still investigating surgical ways to alter the cornea and improve vision once presbyopia begins limiting patients’ range of vision.
One novel approach developed by Soosan Jacob, MS, FRCS, DNB, director and chief at Dr. Agarwal’s Refractive and Cornea Foundation in Chennai, India, uses a biological tissue implant (rather than a synthetic implant) to create a hyperprolate central cornea that improves near and distance vision. The procedure, known as PEARL (PrEsbyopic Allogenic Refractive Lenticule), places a tissue lenticule—sometimes harvested from a SMILE procedure done on another eye and trephined to form a 1-mm disc—into a 120-µm-deep femtosecond laser-created corneal pocket. Using a tissue implant avoids the problems that can be associated with a synthetic implant, such as corneal melt, fibrosis, opacification and haze. After healing, the implant is invisible, and it’s permeable to oxygen and nutrients moving through the cornea. (You can watch a video of the procedure at youtu.be/8H4Ns1b8L3M.)
“I realized there are many issues with putting synthetic implants in the cornea,” says Dr. Jacob. “They were giving good results, and people were happy with the vision they gained, but the cornea never tolerates a synthetic implant very well. I wanted to find a way to provide the advantages of this approach without the complications. That’s when it struck me that if we could do the same thing using biological tissue, these issues might not arise. In fact, that has turned out to be correct.”
Dr. Jacob points out that the tissue doesn’t have to come from a SMILE procedure. “PEARL can be done using any allogenic tissue, processed or unprocessed,” she explains. “Just as LASIK encompasses various different types of ablation profiles and machines, PEARL is similarly an all-encompassing term. We had SMILE tissue available when developing the procedure, so we used that. However, one can use tissue from any source to perform PEARL. In fact, I’m now developing another technique using allogenic tissue to address keratoconus and ectasia, called CAIRS (Corneal Allogenic Intrastromal Ring Segments).”
Dr. Jacob notes that in comparison to some surgical techniques, such as a corneal surface ablation, PEARL is reversible. “Also, it’s an additive procedure, not a subtractive procedure like LASIK,” she says. “In addition, PEARL doesn’t have contraindications such as a thin cornea.
“Of course, no presbyopic solution is perfect,” she adds. “PEARL can cause a slight drop in distance visual acuity, so it’s only done in the non-dominant eye; that way, binocular vision remains good for both distance and near. Patients who might not be good candidates for PEARL would include patients with lens changes, those unwilling to accept a slight drop in distance vision in the operated eye, patients with unrealistic expectations, and patients with systemic or ocular diseases. In terms of possible complications, the cornea could theoretically reject the implant, but we haven’t seen this in our series of cases. Reasons for the low risk of rejection are many, including the position of the implant, the sequestered location and the small volume of tissue used, among others.”
• Supracor. Another approach to overcoming the vision limits associated with presbyopia has been using laser ablation to create a multifocal cornea. One current technique for doing this is the Supracor treatment developed by Bausch + Lomb and manufactured by Technolas Perfect Vision (not currently approved in the United States). The procedure is performed on the Technolas TENEO 317 Model 2 excimer laser (also not available in the United States). Robert E. Ang, MD, a senior consultant at Asian Eye Institute in Makati City, Philippines, and an investigator for Bausch Health Companies, is well-acquainted with the procedure.
“Supracor creates a varifocal cornea,” he explains. “Negative spherical aberration is fashioned in the central 2 mm for near vision, and an aberration-optimized zone is created in the mid-periphery to allow good distance vision. This treatment profile provides approximately 1.5 D of reading add, but since the target refraction is -0.5 D, the result is a total reading add of about 2 D. When a refraction of -0.5 D is achieved, both mean uncorrected near and distance vision are 20/25. Six year data on Supracor treatments for hyperopes and myopes have confirmed this, with a mean MRSE of -0.52 D for hyperopes, and a mean MRSE of -0.45 D for myopes.”
Dr. Ang notes that Supracor is similar to other corneal presbyopia treatments in that it uses the excimer laser to manipulate spherical aberration. “Supracor alters the spherical aberration in the central 2 mm of the cornea,” he says. “By using the center-near, periphery-distance approach, Supracor makes use of pupil dynamics during accommodation, as the pupil constricts when reading and dilates when looking at distant objects. The main difference between Supracor and other corneal ablation approaches is the proprietary Supracor algorithm that determines the amount of aberration change centrally and the optimization of the mid-periphery. The algorithm ensures that distance vision isn’t significantly affected.”
Dr. Ang explains that Supracor is a successor to the IntraCor femtosecond laser procedure. “In IntraCor, five concentric rings were created in the corneal center,” he says. “The rings induced negative spherical aberration through a controlled curvature change and steepening of the central cornea. IntraCor topographies were analyzed and recreated using excimer laser treatment on the stromal bed. Supracor LASIK was found to be better than IntraCor because the excimer laser is very precise in correcting refractive error, targeting a desired refractive outcome and creating a desired corneal shape.”
Dr. Ang points out that Supracor has three different modes: mild; regular; and strong. “To balance distance and near vision and conform to a patient’s needs and expectations, surgeons can choose to utilize a modified monovision approach,” he says. “For example, you might choose to use Supracor mild in the dominant eye and Supracor regular in the non-dominant eye. Or, you could perform standard LASIK in the dominant eye and Supracor strong in the non-dominant eye.”
To ensure a good outcome, Dr. Ang says surgeons have to be careful to center the treatment at the visual axis. “If the treatment isn’t centered, the result can be significant coma, leading to distorted vision and an unhappy patient,” he says. “It’s also crucial to evaluate patient acceptance and manage expectations when selecting patients. Patients have to understand that distance vision after Supracor will probably not be as sharp, which would not be the case with standard, non-presbyopic LASIK.”
In terms of contraindications, Dr. Ang says standard LASIK criteria apply, such as leaving a residual stromal thickness of at least 250 µm and excluding corneas that might have forme fruste keratoconus. “There’s an additional exclusion criterion that applies to hyperopes,” he notes. “If the projected keratometry after Supracor will go beyond 48 D, the patient has to be disqualified because overly steep corneas produce poor outcomes.”
Bausch + Lomb is not currently planning to seek approval for Supracor in the United States.
• VisAbility Micro-Insert. Among the surgical options that may help to address the symptoms of presbyopia is the VisAbility Micro-Insert system (Refocus Group, Aliso Viejo, California). This system consists of four 5-mm-long micro-thin polymethylmethacrylate (PMMA) scleral implants, each smaller than a grain of rice. The implants are placed just below the surface of the sclera about 4 mm from the limbus in the four quadrants of both eyes using a specially modified sclerotome to ensure uniformity. (See illustration above.) Each implant has two parts; the second, smaller part locks the first part in place inside the scleral tunnel.
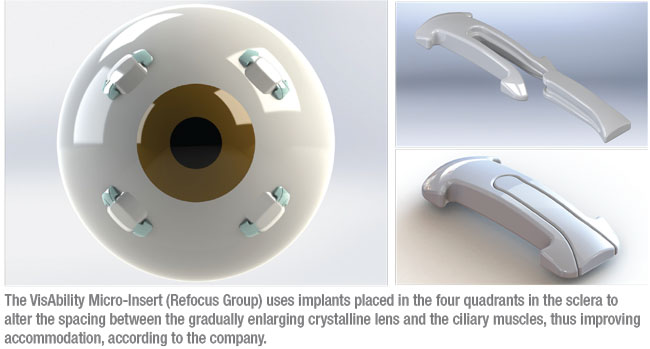 |
The procedure’s mode of action is based on the theory that a key cause of presbyopia is the gradual enlargement of the lens, leading to decreasing space between the lens and the ciliary muscle.2 The VisAbility system is intended to adjust the tension of the posterior zonules, which connect the lens to the ciliary body, thus restoring some amount of accommodation. Placing the implants is an outpatient procedure done under topical anesthesia. Once in place, the company says the implants aren’t felt by the patient or visible to others under normal gaze.
In the initial study, 330 presbyopic subjects age 50 to 60 had the procedure in the primary eye, followed by surgery in the fellow eye at least 14 days later. The primary endpoint was to have at least 75 percent of patients achieve binocular 20/40 (or J3) DCNVA at 40 cm by the end of the study. At one year, 94 percent had achieved this, and DCNVA continued to improve during three years post-surgery. At the 2017 meeting of the American Academy of Ophthalmology, James Katz, MD, reported data from 177 participants who were followed-up for 36 months. Ninety-five percent of subjects had achieved the endpoint by 24 months; the percentage achieving this rose to 97 percent at 36 months.
Most potential downsides associated with the procedure appear to relate to healing and potential pain from the scleral surgery.2
A follow-up study is currently under way to obtain an additional 36 months of safety and effectiveness data from subjects implanted with the inserts during the previous trial. The company hopes to complete this trial by July 2021. The VisAbility Micro-Insert system is currently under FDA consideration.
Altering the Lens or Cornea With Pharmaceuticals
While some novel pharmaceutical options for presbyopia focus on the pupil, others take a different route: the crystalline lens or the cornea. Here’s an update on their status.
• Novartis’ UNR844 Drop. Researchers have long noted that presbyopia appears to result from multiple factors, one of which is reduced crystalline lens flexibility with aging. Evidence suggests that our lenses gradually oxidize, causing disulfide bonds to form. These disulfide bonds restrict the lens’s ability to change shape in response to ciliary muscle contraction and relaxation, and they contribute to the development of nuclear sclerotic cataracts. Thus, one potential approach to treating presbyopia is chemically reducing disulfide bonds in the lens.
A topical agent from Novartis, referred to as UNR844, uses lipoic acid choline ester to do exactly that. The drug is formulated as a prodrug that improves its penetration through the cornea. The drop is intended for bilateral use.
The company’s prospective, randomized, double-masked, placebo-controlled Phase I/II study enlisted 75 patients age 45 to 55 years with a diagnosis of presbyopia. At baseline, participants’ distance-corrected near vision was below 20/40 in both eyes. Fifty patients received UNR844; 25 received placebo. Patients received drops for 91 days and were then monitored for seven months. Findings included:
• Bilaterally, 84 percent of patients receiving UNR844 improved to 20/40 or better, versus 52 percent of those receiving placebo.
• Fifty-three percent of those receiving UNR844 experienced an improvement of at least 0.2 logMAR; only 22 percent receiving placebo achieved this.
• The drug caused no change in visual acuity, manifest spherical equivalent or pupil diameter.
• Seven months after study completion, 39 percent of treated subjects maintained their improvement in bilateral vision, compared to only 6 percent of the placebo group.
• No subjects discontinued.
“One of the advantages of this approach to treating presbyopia is that its mechanism of action directly addresses the cause of presbyopia and nuclear sclerotic cataract,” says Michael Korenfeld, MD, an associate clinical professor at Washington University School of Medicine, and a principal investigator and paid consultant for Novartis. “Currently, we don’t know how much restoration of function can be accomplished with this drug. The recent study included a small cohort of humans dosed for 90 days. In theory, it might be possible to achieve total restoration of accommodative function with longer durations of treatment or more frequent intervals of dosing, but at this point we simply don’t know.”
Dr. Korenfeld notes that there’s some debate about whether addressing the root cause of presbyopia is a better approach than relieving symptoms temporarily. “The effects of this drug last a long time,” he points out. “Some companies are developing drugs that make the pupil smaller for less than a day. They see being able to turn near vision improvement on and off as a benefit. Others think that reversing the underlying pathology and having a lasting and continuous benefit is more advantageous.” (Of course, UNR844 only works if the patient still has his natural lens; a pupil-based solution doesn’t have that limitation.)
Dr. Korenfeld says that, with the exception of pseudophakic patients, he sees no obvious reason this couldn’t be tried on any presbyopic patient. “Novartis is actively working to develop this drug,” he notes. “At this point it’s impossible to say when it might reach the marketplace, but it will probably be within a few years.”
• Yolia True Vision. Another approach to addressing the limited vision caused by presbyopia, using a system originally developed in Mexico by the late Alberto Osio Sancho, MD, and later incubated at Johnson & Johnson Labs, is now available in Mexico from Yolia Health. According to Alberto Osio, CEO of Yolia Health, True Vision is a non-invasive, repeatable treatment involving patient self-administration of proprietary enzyme eye drops that increase corneal malleability, followed by wearing individually customized contact lenses that reshape the cornea’s sphericity to produce multifocal vision. (TVT is approved by the Mexican FDA; it’s part of Yolia Health’s “Invisalign” platform of treatments using the same premise to correct a range of vision problems including myopia, hyperopia, digital fatigue and post-surgery refractive errors.)
Patients use the TVT system for a week; the company says that the resulting vision change can last for up to a year before retreatment is needed. It’s designed to be used in both eyes, improving near vision without compromising distance vision. According to the company, after using TVT, 92 percent of patients can read newsprint, with a near-vision gain of up to five lines lasting more than eight months, and 91 percent report improved distance vision as well. TVT can be repeated as needed, and the reshaping can be modified over time as the eyes change. The company reports no adverse effects associated with the treatment.
A small study sponsored by Yolia enrolled 50 patients between the ages of 40 and 60. Participants were screened for dry-eye disease and their vision and anterior segments were assessed; then contacts lenses were prepared and TVT kits were distributed to each subject. Findings included:
• At day eight, uncorrected near visual acuity was significantly improved in all eyes (p<0.01), with an average near gain of more than two lines.
• Seventy percent of patients had an improvement of two lines, almost half had an improvement of three lines, and 23 percent had an improvement of four or more lines.
• Uncorrected distance acuity also improved an average of one line by day eight (p<0.01).
• These improvements were stable from day eight to day 180.
More than 600 patients, including 55 international patients, have been treated with the TVT system. TVT has been submitted to the FDA, and the company hopes to receive approval by 2023. REVIEW
1. Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: A prospective, consecutive interventional non-comparative clinical study. Ophth and Therapy 2019;8:31-39.
2. Baitch L, Schanzlin D, Iskander D. One-year post-operative wavefront and refractive map changes following scleral implant surgery. Presented at the 15th International Congress on Wavefront & Presbyopic Refractive Correction, 2014; Dana Point, California.