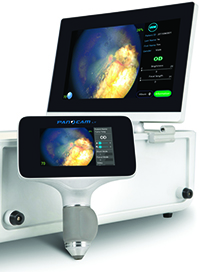
 |
PanoCam LT is a compact, wireless imaging system that is designed to detect a number of external, anterior and posterior segment vision disorders that may have long-term effects on the vision of millions of children worldwide each year. Clinical data suggests that vision disorders may affect between 10 and 20 percent of all newborns globally.
“We are launching the Panocam LT, the first completely wireless, hardware and software solution, to help ophthalmologists and maternal clinicians identify vision disorders in newborns. Our PanoCam LT and the PanoCam Review Software will help clinicians identify newborn vision disorders,” that, if treated earlier, may preserve healthy vision, says Visunex CEO, Dr. Wei Su.
An estimated 4 million children in the United States and 130 million globally are born each year. Vision screening is not currently provided as standard of care in most neonatal centers. Research conducted in Asia, Brazil and the United States suggests that one in 70 newborns may have some form of vision disorder.
Most commonly, the first eye test performed on a child is prior to kindergarten at the age of 5 or 6. While many visual disorders may be transient, a growing number of clinicians believe that the screening of newborns may provide early detection of vision disorders, such as retinal hemorrhages, which may be a precursor to amblyopia. Early detection of retinal hemorrhages as well as many other serious disorders can lead to early therapeutic intervention and potentially prevent vision loss. For more information, visit visunexusa.com.
Ziemer Femto Laser Approved
The FDA also granted 510(k) approval for use of the Ziemer Femto LDV Z8 in the United States.
Ziemer says the Femto LDV Z8 offers the first truly mobile femtosecond laser solution for refractive, cornea and cataract procedures to be performed on an all-in-one-system. The laser uses unique technology that provides precision in the cornea and power in the lens with use of adjustable pulse energy combined with a high repetition rate to provide a precise capsulotomy and lens fragmentation, the company reports. The innovative, compact design provides a mobile and versatile workstation made for seamless integration into any physician office or operating room in the country. The Femto LDV Z8 is approved for Z-Lasik and Z-Lasik Z; intracorneal rings; intrastromal pockets; lamellar keratoplasty; penetrating keratoplasty; anterior capsulotomy; lens fragmentation; and clear corneal and arc incisions. For information, visit femtoldv.com or ziemerusa.com.
Multi-Dose Restasis Sought
Allergan has submitted a Prior Approval Supplement (PAS) for Restasis (cyclosporine ophthalmic emulsion) 0.05%, seeking approval of a multi-dose, preservative-free presentation. If approved, the presentation would offer patients the same formula in a multi-dose system with patented uni-directional valve and air filter technology.
Approved by the FDA in 2002, Restasis is the only eye drop that helps increase the eyes’ natural ability to produce tears, which may be reduced by inflammation due to chronic dry-eye disease. Restasis did not increase tear production in patients using anti-inflammatory eye drops or tear duct plugs. REVIEW



