"Basically, the technology looks at the eye at the WaveScan, and then again underneath the laser," explains John Weberg, senior global marketing manager for laser technologies at Visx. "Taking the different exam environments into account, such as the varying lighting conditions, sophisticated algorithms pick out 48 highly defined areas of iris crypts between the WaveScan and the Star S4 IR laser for matching purposes." To activate registration, the surgeon presses a button once the flap is lifted and the tracker is engaged.
Mr. Weberg says that, to help avoid false readings, the registration software has very strict protocols that look for any false matches. There's also a significant number of positive matches that it must detect, which he says yields a high degree of accuracy in matching the iris with the correct wavefront map. Once it confirms registration, the laser then adjusts each individual pulse by the amount of cyclotorsion that it calculated for that eye.
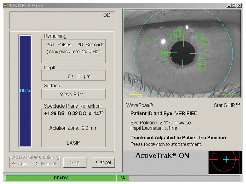
"In the studies we've done, most physicians are accurate up to approximately 5 degrees of cyclotorsion with their marking techniques," says Mr. Weberg. "But even at 5 degrees, that can be fairly significant, depending on the patient's eye, especially in higher amounts of astigmatism. This is significantly more accurate than manual marking." He says there will be papers at the American Society of Cataract and Refractive Surgery on the visual outcomes of registered vs. unregistered procedures.
Chicago refractive surgeon and Visx investigator Colman Kraff has used the iris registration system initially in a Visx trial he is currently involved with, and has now been using it commercially for a couple of weeks. He doesn't have any clinical results from its use, yet, however.
"So far it's been very reliable," says Dr. Kraff. "I think it's going to improve outcomes in some patients. We currently don't know how much rotation will be significant. We'll learn by treating patients with this iris-registration platform what significant cyclorotation is in a CustomVue platform."
Visx's Mr. Weberg says that the upgrade consists of both hardware and software upgrades to the laser. In order to use iris registration, surgeons need to have the Fourier upgrade on their WaveScan aberrometer. The hardware for the laser involves a new camera, new covers that fit around the laser head, and new PC boards.
The upgrade does have a cost, which the company isn't making public at this time. For pricing information, users can call 1 (800) 246-VISX.
Survey Identifies Issues Important to Cataract Patients
The reports of changing attitudes and demands of the next generation of cataract patients have some new support in the form of a recent Nielsen consumer survey.
In November 2004, A.C. Nielsen BASES surveyed 322 patients aged 55 to 75, with a valid driver's license, who had not undergone cataract surgery, and who drive at night at least once every few weeks. Commissioned by Advanced Medical Optics, the survey focused on the issue of gradual loss of vision due to cataracts, in relation to the respondents' level of concern about: maintaining certain functional activities such as watching television, reading, participating in hobbies or crafts, and driving; and the importance of intraocular lens performance in maintaining or enhancing visual function.
Mobility and independence are of paramount concern to the older population. Of the 252 respondents in the survey who drive a vehicle everyday, 90 percent said they were "extremely" or "very" concerned about not being able to drive, especially at night. Overall, 88 percent of adults between 55 and 75 rated this issue "extremely" or "very" important.
Cynthia Owsley, PhD, MSPH, director of the Clinical Research Unit at the Callahan Eye Foundation Hospital, in Birmingham, Ala., says, "Most older adults in this country do not live in situations where there's adequate and accessible public transportation or transportation services. The vast majority of Americans don't live in those situations where there are communities built where there are little shuttle services, your stores and medical services within your community… [Older adults] want to be able to see to drive because driving is such an important activity of daily living that's really very intimately tied to quality of life."
The survey also showed that the desire to have a lens implant that would improve quality of vision, particularly for driving and improved driving safety, was important to patients with cataracts or facing cataract surgery.
In another question focusing on driving issues, 91 percent surveyed said that it was "extremely" or "very" important to have a lens that provides a quality of vision that improves reaction time, stopping time, and stopping distance when driving. Additionally, 91 percent also felt it was "extremely" or "very" important to have a lens that improves the ability to see in both bright light and low light situations.
Ralph Chu, MD, of the Chu Laser Eye Institute, in Edina, Minn., implants AMO's Tecnis Z9000 aspheric IOL. "When I saw the Tecnis FDA data show that basically you're not only improving Snellen acuity like a typical IOL, but you're improving quality of vision, contrast at night, to the point where the FDA felt that they had to put an indication on the labeling that said 'improved safety for night driving', that was amazing," he says. "Now you have an implant that actually was approved for improving safety, with data to back it up." The Tecnis is only lens to have such an indication from the FDA.
Eighty-two percent of the 322 patients surveyed said FDA approval of a lens to improve night driving performance was an important factor to them. Dr Chu was not surprised at the finding and thought it was among the most significant. "Patients want their care based on science," he says. "They want to see that it's not just a marketing thing, and so they want to see that there's data to back it up."
Another question in the survey focused on the importance of a new lens implant for treating cataracts to restore the eyes' function to that of a healthy young adult, not a healthy older adult. Eighty-nine percent of respondents, 88 percent of whom drive daily, rated this the most important benefit of a new lens implant.
"[G]iven our research on cataract in older drivers and cataract surgery and crash involvement, this would not be surprising," says Dr. Owsley. "If you ask an older adult, do you want a lens that will let you see like a 60-year-old normal older adult without a cataract or like you saw when you were 30, it's sort of a no-brainer."
Brookville, Pa., surgeon Louis D. Nichamin, MD, agrees. "Just as we've seen with kerato-refractive surgery, where now with custom ablation and wavefront aberrometry we talk only about quality of vision, we can get 20/20 on the vast majority of patients with LASIK and indeed we can even do that with IOL surgery," he says. "The next bastion, the next level, are these issues of subtle qualities, and I think we're just beginning to broach that now with IOL surgery, but that's going to be the future." Dr. Nichamin has been implanting the Bausch & Lomb SoftPort AO aspheric lens since its approval last year, and was an investigator in the lens's FDA trials.
Dr. Owsley believes the profession often "thinks in terms of cataract surgery as giving the patient better vision to read or better vision to pass the acuity test to maintain their [driver's] license," but doesn't necessarily "think about the larger ramifications of having better contrast sensitivity, or improving their mobility function."
All three doctors agree that the newer cataract patient is younger, living longer, and is much more discriminating and demanding. These patients continue to drive, play golf, go to the movies and overall lead active lives well into their 70s and 80s. This had led to a new perception of cataract patients among some surgeons.
Says Dr. Chu, "The basic change has been that we treat every cataract patient as a refractive patient, so we're actually giving a complete assessment of the optical system. We're doing topographies, wave scans, a broad assessment of their eyes, not just a standard cataract evaluation, 'You've got a cataract, let's take it out.' We're actually thinking of them now as refractive patients."
The new challenge is to get cataract surgeons to think beyond the technique of the cataract extraction procedure and begin to think more broadly about the other potential optical outcomes. However, observers suggest that the profession needs to develop a more heightened awareness of the potential of the aspheric IOL if it wants to provide high-quality cataract surgery results.
Researchers Identify Important AMD Gene
Scientists at Rockefeller University, Yale University School of Public Health and the National Eye Institute have identified a gene that confers susceptibility to age-related macular degeneration. Reported in the March 10 issue of Science Express, the finding opens the door for new investigations of the role of genes in developing AMD and possible treatments for this disease.
"We have shown that a variant, or polymorphism, of the complement factor H gene, which alters a protein whose normal function is to regulate the immune system's attack of foreign invaders and abnormal cells, is involved in the development of AMD," says senior co-author Jürg Ott, PhD, professor and head of the Laboratory of Statistical Genetics at Rockefeller. "We believe this polymorphism is a strong risk factor for the disease."
The gene variant, known as a single nucleotide polymorphism (SNP), derives from a single letter difference in the genetic sequence of DNA. Some of these differences may change a gene's protein products in ways that may confer susceptibility, or protection from, diseases. In this case, the complement factor H (CFH) SNP associated with AMD encodes for a different amino acid, as histidine substitutes for tyrosine at a specific position. The CFH gene lies in a region of human chromosome #1 that had been linked previously to AMD through family studies by other researchers.
For the research reported in Science Express, Josephine Hoh, PhD, an assistant professor at Yale's School of Public Health, used DNA taken from blood samples collected for the National Eye Institute-sponsored Age-Related Eye Disease Study. AREDS was designed to learn more about the natural history and risk factors of AMD and cataract and evaluate the effect of high doses of antioxidants and zinc on the progression of these conditions.
Dr. Hoh and colleagues analyzed DNA from 96 unrelated patients with an advanced form of AMD and 50 healthy people who had little or no drusen deposits in their retinas.
The controls were chosen to be older than those with AMD. The study was carefully designed by matching other potential factors such as ethnicity, gender and smoking to ensure the only differences between the two study groups was disease status and genetic background.
The researchers genotyped more than 116,000 SNPs using the most advanced microarray technology and compared the frequency of each of the 116,000 SNPs in the two groups, patients and controls.
Biochemical analysis of drusen by other researchers has shown that the deposits are largely composed of lipids, but a small portion of the drusen are components of the immune system called complement. The complement system is a collection of related proteins that are the body's front-line defense system, the innate system, that attacks foreign invaders while usually avoiding any attacks against healthy cells. And one of the known properties of factor H is that it regulates the activation of complement components.
The researchers examined the eyes of four patients with AMD and found complement debris in the drusen, as well as in Bruch's membrane and the intercapillary pillars. Other researchers also have detected complement components in the drusen of humans.
"The polymorphism produces a change in a specific amino acid in the complement factor H protein, which is located in the region that interacts with C-reactive protein and heparin," says Dr. Hoh. C-reactive protein is associated with heart disease and high cholesterol levels and both C-reactive protein and heparin are associated with AMD.
Alcon Retaane Review Moves Forward
Alcon reported the results of a clinical pharmacokinetic study evaluating the effectiveness of a counter pressure device (CPD) the company developed to control reflux during the administration of Retaane 15 mg (anecortave acetate for depot suspension) by posterior juxtascleral depot (PJD). The results of this PK study demonstrated that the CPD was effective in controlling drug reflux in 100 percent of the study participants. Reflux occurs when a portion of the drug leaks back out through the small incision in the conjunctiva during or immediately following the PJD procedure. Henry L. Hudson, MD, of Tucson, Ariz., and Donald Roy, MD, of Fresno, Calif., conducted the study. Allen Ho, MD, of Philadelphia, presented the data at last month's Macula Society Meeting in Key Biscayne, Fla.
In addition to measuring reflux, the study also measured the concentration of drug in blood plasma to confirm that effectively controlling reflux correlated with a higher level of drug absorption. The results of this study supported this relationship, as the patients in this study had higher concentrations of drug than did patients in previous trials who experienced drug reflux. These data establish that PJD administration of Retaane depot using the CPD results in effective delivery of the drug.
"With Alcon's new counter pressure device, reflux was effectively controlled during administration of Retaane in Dr. Hudson's and Dr. Roy's study," said Dr. Ho. "These positive results clearly demonstrate that Alcon has successfully resolved drug reflux. Now physicians and patients can expect to have a sufficient amount of drug to last for the entire six-month treatment interval." Stella Robertson, PhD, vice president of ophthalmic research and development at Alcon added, "This study responds to the FDA's specific request that we demonstrate that reflux can be controlled with the counter pressure device, and its success should mitigate any concern about reflux. We know from our phase III data that 57 percent of patients who experienced no reflux and were treated with Retaane within six months maintained their vision, compared to 49 percent of patients treated with Visudyne; and these results met the 7-percent non-inferiority standard originally assigned to the trial. Furthermore, the overall results of our Phase III trial meet a 14 percent criterion more specific to the patients evaluated in the study."
In addition, the company announced the FDA accepted its New Drug Application for Retaane depot as fileable and has confirmed a priority review assignment. Based on the date of its submission, Alcon expects an FDA decision in late May.



