In healthy eyes of young patients, the vitreous is a clear gel that fills the vitreous cavity, occupying approximately 80 percent of the volume of the globe. The vitreous consists mostly of water (99 percent) as well as hyaluronic acid and a meshwork of fine collagen fibrils. An important area is the vitreous base, a 3- to 4-mm-wide circumferential zone of vitreous that straddles the ora serrata. In the vitreous base, the collagen fibers are firmly attached to the underlying peripheral retina posterior to the ora and to the pars plana epithelium anteriorly. Other areas of firm vitreous attachment are at the edges of retinal scars, in areas of lattice and other vitreoretinal degenerations, at the optic disc, and along the major vascular arcades.
Posterior vitreous detachment (PVD) occurs when the posterior vitreous separates from the retina and collapses anteriorly toward the vitreous base. This event occurs as a result of central vitreous syneresis (liquefaction). With increasing age, an opening ultimately develops in the posterior vitreous through which the central liquefied vitreous passes suddenly into the retrovitreal space, rapidly dissecting a plane and separating the posterior hyaloid from the retina.1,2
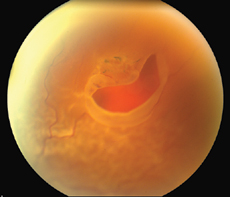 |
| Figure 1. A large horseshoe tear with retinal detachment. |
Posterior vitreous detachment is the critical event leading to the development of retinal tears and retinal detachment. When the separating vitreous remains firmly adherent to an area of retina, localized vitreoretinal traction results. PVD may produce retinal breaks in areas of firm vitreoretinal attachments and on narrow posterior extensions of the vitreous base. Mild traction causes tenting of the retina. With additional traction a horseshoe tear (flap tear) occurs (See Figure 1). With still further traction, the flap may separate from the surrounding retina, leaving a round or oval hole with an overlying operculum (See Figure 2).
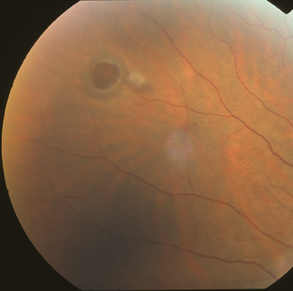 |
| Figure 2. Round hole with a cuff of subretinal fluid and an overlying operculum. |
Retinal breaks, especially horseshoe tears caused by acute vitreous detachment, can result in retinal detachment by allowing liquid vitreous to enter the subretinal space and separate the sensory retina from the retinal pigment epithelium. Patients at risk for retinal detachment include those with high myopia, aphakia, detachment in the fellow eye, and family history of detachment. Peripheral retinal lesions that predispose to retinal detachment include lattice degeneration, vitreoretinal tufts, meridional folds, enclosed ora bays, and peripheral retinal excavations.
Ten to 15 percent of patients with acute symptomatic PVD are found to have a retinal tear.2,6,7,8,9In many of these patients, pigment epithelial cells migrate through the tear and can be seen dispersed throughout the vitreous. The presence of peripheral punctate intraretinal hemorrhages often indicates vitreous traction and may mark the site of a future tear.9,10 Vitreous hemorrhage is another important sign. When present, there is a 70-percent incidence of retinal tears compared with a 2- to 4-percent incidence without hemorrhage.11 Hemorrhage results from avulsion of superficial retinal or prepapillary vessels or from torn retinal vessels that cross retinal tears. In some cases, however, an intact vessel may cross over a retinal tear, and continued vitreoretinal traction on the bridging vessel may cause vitreous hemorrhage to occur later.12
History and Examination
Typical symptoms of PVD are "flashes and floaters." Photopsia, the subjective impression of flashing lights, is caused by vitreous traction on the peripheral retina. Floaters are described as cobwebs, spots or hair in the field of vision, and they are caused by vitreous opacities such as epipapillary glial tissue torn from the optic disc, condensations of vitreous collagen, and/or blood. There are no symptoms that can distinguish PVD alone from PVD with an associated retinal break. If an associated retinal detachment is present, patients may complain of a "curtain" or "shadow" to describe loss of visual field.
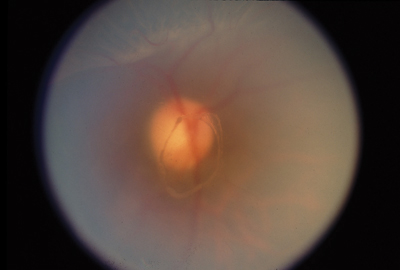 |
| Figure 3. Round hole with a cuff of subretinal fluid and an overlying operculum. |
Slit-lamp examination may demonstrate pigmented cells in the anterior vitreous (tobacco dust, or Shafer's sign) and/or vitreous hemorrhage. As noted earlier, either of these findings increases the likelihood that a retinal break occurred with the PVD. Slit-lamp biomicroscopy using a 90-D lens enables the examiner to visualize the posterior vitreous overlying the posterior pole.
The diagnosis of posterior vitreous detachment is typically made when a ring of glial tissue is seen attached to the posterior hyaloid anterior to the optic nerve head (Weiss ring). This ring has the same diameter of the optic nerve head (approximately 1.5 mm) since it represents epipapillary glial tissue torn from the edge of the optic disc (See Figure 3). Although a Weiss ring usually indicates that a total posterior vitreous separation is present, vitreous may rarely remain attached to the macula or other posterior structures despite its separation from the optic nerve head.
A binocular view of the retinal periphery is essential, and this is obtained using contact lens biomicroscopy or indirect ophthalmoscopy with scleral depression. Direct view contact lenses, such as the Goldmann three-mirror lens, provide excellent magnification without image inversion. In contrast, wide-field contact lenses provide panoramic views with less magnification and image inversion. In phakic eyes, prominent cortical spokes may interfere with peripheral retinal visualization using contact lens biomicroscopy. Similarly, in pseudophakic eyes, anterior or peripheral posterior capsular opacification, retained cortex within the capsular bag, or the intraocular lens edge may limit the peripheral fundus examination.
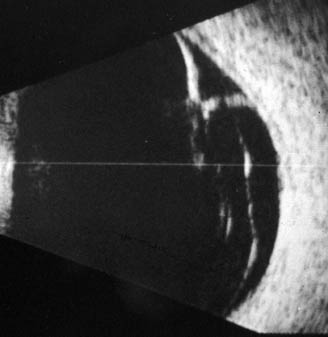 |
| Figure 4. B-scan ultrasound demonstrating peripheral retinal tear and localized retinal detachment in a patient with PVD and vitreous hemorrhage. |
Indirect ophthalmoscopy uses hand-held lenses with variable dioptric powers. Lenses with greater dioptric power provide greater field of view but less magnification. The 20-D and the 28-D lens are the most commonly used lenses for indirect ophthalmoscopy, with the 28-D lens being particularly useful for evaluation of gas-filled eyes and eyes with a small pupil or a contracted and/or opacified capsule. A small-pupil indirect ophthalmoscope is also useful in this situation.
Indirect ophthalmoscopy is best performed with the patient reclined and the chair raised. There should be space for the examiner to be able to freely move around the patient's head. One should stand to the right of the patient's head when examining the peripheral nasal 180 degrees of the right eye and the peripheral temporal 180 degrees of the left eye. Similarly, one should stand to the patient's left side to evaluate the remaining hemispheres.
Scleral depression must be performed in all patients with symptoms or signs of vitreous or retinal detachment. A metal scleral depressor or a cotton-tipped applicator can be used to indent the globe through the patient's lids. Although scleral depression may induce anxiety in some patients, only minimal pressure is needed, and the technique should produce minimal to no discomfort. The eye should preferably be almost in primary position. In the extremes of gaze, the ora is rotated posteriorly and one has to push more posteriorly in the orbit to see the depression. Excessive pressure, or pressure applied too posteriorly, will result in patient discomfort, anxiety and lid squeezing.
The depressor is typically held in the hand closest to the area being examined. For example, when standing to the patient's right side while examining the nasal 180 degrees of the right eye, the depressor is held in the left hand to view the superonasal periphery (to depress the upper lid), and it is held in the right hand to view the inferonasal periphery (to depress the lower lid). Scleral depression involves indentation of the globe to push the peripheral retina into view, and it allows the retina to be seen in tangential profile. Movement of the depressor enables a dynamic evaluation to better expose peripheral pathology, as minute changes of retinal contour may be visualized.
Since retinal breaks may be only a few microns in size, the entire 360 degrees of peripheral retina must be compulsively examined, with particular attention directed to the posterior edge of the vitreous base. (The equatorial region must also be examined, for it is possible to examine the posterior pole and then the periphery, inadvertently omitting a relatively wide area of equatorial retina in between.)
A peripheral retinal horseshoe tear is recognized as the retinal flap rises from the crest of the indented retina. A retinal hole appears as a deep reddish spot with an overlying retinal defect, and an operculum may be seen adherent to the mobile posterior vitreous. An area of retinal detachment is identified by retinal elevation that may undulate with eye movements, outer retinal hydration lines, and/or loss of underlying choroidal detail.
Ultrasound
In the presence of significant media opacity or insufficient pupillary dilation that preclude adequate fundus visualization, posterior segment structures must be imaged with ultrasound when peripheral retinal pathology is suspected. Ultrasonography involves high-frequency sound waves that are propagated through tissue media and interact with ocular structures of varying size and density. A transducer records the wavefront interactions to produce a wave form (A-scan) or a two-dimensional cross-sectional image (B-scan).
In B-scan ultrasonography, ocular structures are viewed in real time with 300- to 400-µm resolution. Posterior vitreous detachment can be diagnosed using B-scan ultrasound even in the presence of dense vitreous hemorrhage. Anterior structures such as the peripheral retina and ciliary body are not particularly well-imaged using this technique; however, peripheral retinal breaks (horseshoe tears) can sometimes be demonstrated by a skilled ultrasonographer (See Figure 4).
Retinal detachment can be diagnosed using a dynamic study with combined A- and B-scan modalities. If vitreous hemorrhage is present and ultrasound fails to demonstrate a retinal tear or detachment, bed rest with the head of the bed elevated 45 degrees for one to two days may clear the vitreous enough to permit adequate visualization.13
Treatment
Patients with acute symptomatic (floaters and/or photopsia) PVD without vitreous hemorrhage or peripheral retinal breaks require no immediate treatment but may be re-examined in one to two weeks, since some retinal breaks appear to develop days to weeks after the onset of symptoms. The development of retinal detachment is unlikely if no retinal tears are present at follow-up in four weeks.8 Patients with peripheral punctate intraretinal hemorrhage should be followed more carefully, as should patients with vitreous hemorrhage. All patients are counseled and instructed to return immediately if they have a significant change in symptoms, such as new floaters, which might indicate progression of PVD with a retinal tear.
Acute symptomatic retinal breaks resulting from PVD have a high risk of retinal detachment with potential permanent visual loss. Most retinal specialists treat acute symptomatic horseshoe tears, and even symptomatic operculated breaks, in an attempt to prevent retinal detachment.11,14 Twenty-five to 90 percent of acute symptomatic horseshoe tears have been found to cause retinal detachment, and treatment reduces the frequency of subsequent retinal detachment to 5 percent.
The goal of treatment is to create a chorioretinal scar surrounding each break so that no communication between the vitreous cavity and subretinal space exists. Either cryopexy or laser can be used; however, most retinal specialists prefer laser. Cryopexy is more uncomfortable than laser and usually requires some form of injectable anesthesia. In addition, there is more dispersion of retinal pigment epithelial cells after treatment with cryopexy.
Cryotherapy is most beneficial in cases in which media opacity precludes transmission of laser energy. Barrier laser is delivered at the slit lamp using a contact lens or with the laser indirect ophthalmoscope using scleral depression. Three to four rows of a very closely spaced, almost confluent pattern of laser spots, 500 µm in size, are usually sufficient (See Figure 5). Breaks should be completely surrounded, and for horseshoe tears, treatment must extend further anteriorly, preferably to the ora serrata because persistent vitreous traction can pull the flap through a cryopexy or laser scar and cause retinal detachment.
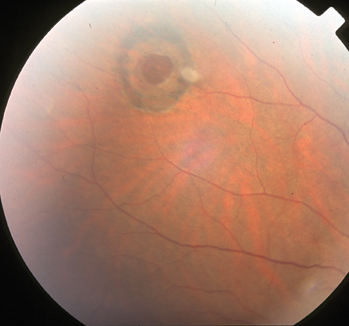 |
| Figure 5. The retinal hole in figure 2 six months after laser treatment. |
Patients should also be informed that peripheral visual loss may be an early symptom of retinal detachment, and they should report immediately if they perceive this or note reduced vision. If patients are familiar with the symptoms of retinal detachment, they are likely to report promptly after its onset, increasing the chances for successful surgical and visual results.15,16 When retinal detachment is present, surgery involves finding and closing all retinal breaks. A firm chorioretinal adhesion is produced by cryotherapy or laser.
The break(s) can be closed by a number of methods. A scleral buckle indents the sclera beneath the retinal break and promotes re-apposition of the retina to the retinal pigment epithelium by reducing vitreous traction and diminishing the flux of vitreous through the retinal break. Pneumatic retinopexy, an alternate technique used in selected retinal detachments with breaks in the superior fundus, an expansile gas bubble is injected into the vitreous cavity to occlude the retinal break until the retina reattaches. Vitrectomy surgery is used in selected retinal detachments to internally relieve vitreoretinal traction.
Approximately 10 percent of patients who develop a retinal detachment in one eye (half with and half without predisposing lesions) will later experience one in the fellow eye. Prophylactic treatment of the fellow eye may be considered, and counseling during periodic follow-up is indicated. Educating patients can positively affect the visual and surgical outcome if detachment develops in the second eye.
Dr. Eliott is the director of the Retina Service and an associate professor of ophthalmology at the Kresge Eye Institute, Wayne State University. Contact him at (313) 993-0871; (fax) 313 577-2905; e-mail: deliott@med.wayne. edu.
1. Foos RY. Posterior vitreous detachment. Trans Am Acad Ophthalmol Otolaryngol 1972; 76:480-497.
2. Lindner B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol (suppl) 1966;87:1.
3. Osterlin S. Vitreous changes after cataract extraction, in Freeman HM, Hirose T, Schepens CL (eds): Vitreous Surgery and Advances in Fundus Diagnosis and Treatment. New York: Appleton-Century-Crofts, 1977:15-21.
4. Heller MD, Straatsma BR, Foos RY. Detachment of the posterior vitreous in phakic and aphakic eyes. Mod Prob Ophthalmol 1972;10:23-36
5. Ober RR, Wilkinson CP, Fiore JV, Maggiano JM. Rhegmatogenous retinal detachment after Neodymium-YAG laser capsulotomy in phakic and pseudophakic eyes. Am J Ophthalmol 1986;101:81-89.
6. Boldrey EE. Risk of retinal tears in patients with vitreous floaters. Am J Ophthalmol 1983;96:783-787.
7. Jaffe NS. Vitreous detachments. In: The Vitreous in Clinical Ophthalmology. St. Louis: CV Mosby Co., 1969:83-98.
8. Tabotabo MM, Karp LA, Benson WE. Posterior vitreous detachment. Annals Ophthalmol 1980;12:59-61.
9. Tasman WS. Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol 1968;72:217-224.
10. Kanski JJ: Complications of acute posterior vitreous detachment. Am J Ophthalmol 1975; 80:44-46.
11. Benson WE. Retinal Detachment: Diagnosis and Management, ed 2. Philadelphia: Harper & Row Publishers, Inc, 1988:4.
12. Robertson DM, Norton EWD. Long-term follow-up of treated retinal breaks. Am J Ophthalmol 1973;75: 395-404.
13.Seelenfreund MH, Sternberg I, Hirsch I, Silverstone B-Z. Retinal tears with total vitreous hemorrhage. Am J Ophthalmol 1938;95:659-662.
14. Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol 92:183-194,1974.
15. Tani P, Robertson DM, Langworthy A. Rhegmatogenous retinal detachment without macular involvement treated with scleral buckling. Am J Ophthalmol 1980; 90:503-508.
16. Wilkinson CP, Anderson LS, Little JH. Retinal detachment following phacoemulsification. Ophthalmology 1978;85:151-156.