Various treatment regimens, such as treat-and-extend and as-needed administration, have been adopted to minimize treatment burden and potentially improve compliance, while also personalizing treatment regimens away from fixed-dosing regimens. However, real-world outcomes of anti-VEGF therapy are generally worse than those obtained in clinical trials, possibly due to undertreatment.3 A large, retrospective analysis of a U.S. medical claims database showed that 19,000 newly diagnosed nAMD patients received a mean of only 4.6 and 6.9 injections of bevacizumab and ranibizumab over 12 months, respectively. A meta-analysis of anti-VEGF treatment regimens for nAMD suggests that there is a positive linear correlation with visual acuity gains and number of bevacizumab or ranibizumab injections over 12 months, although there was a ceiling effect in which more than nine injections annually didn’t result in further gains.4 Theoretically, such a regimen could be arranged as three monthly loading doses followed by a six-week injection interval thereafter, but this regimen would represent a departure from the trend towards personalized medicine and personalized treatment regimens. Aflibercept, which is FDA-approved for three monthly loading doses followed by two-month dosing afterward, can be dosed in as few as eight injections over the first 12 months.
Given the limitations of anti-VEGF therapy, along with the burdensome need for repeated intravitreal injections to sustain efficacy, long-acting formulations of anti-VEGF drugs, as well as topical and oral formulations, are being developed. Brolucizumab and abicipar pegol are two novel intravitreal anti-VEGF treatments that could potentially be approved for 12-week (quarterly) dosing after three monthly loading doses, which would result in six injections over the first 12 months (fewer than all three currently available anti-VEGF agents). However, a deeper dive into the data raises important questions. Some novel anti-VEGF treatments, currently in clinical trials, are summarized below, with a more comprehensive overview in the table.
Long-acting Anti-VEGF Agents
Rather than use a sustained-delivery approach, some agents that are being studied are designed to require less-frequent dosing.
• Brolucizumab. Brolucizumab (Novartis) is a humanized single-chain antibody fragment inhibitor of VEGF-A that has a smaller molecular weight (26 kDa) compared to aflibercept (115 kDa) and ranibizumab (48 kDa). Its molecular properties allow a higher molar concentration to be prepared in a 0.05 ml intravitreal injection, which may allow for an extended duration of effect and improved ocular tissue penetration.
The Phase III HAWK and HARRIER trials (ClinicalTrials.gov identifiers: NCT02307682, NCT02434328) compared aflibercept 2 mg to brolucizumab 3 mg and brolucizumab 6 mg. This study enrolled more than 1,800 patients across 400 centers worldwide. After three monthly loading doses, the brolucizumab groups were treated every 12 weeks, with the option of switching to eight-week dosing in case of recurrent disease activity.5 A Novartis press release from June 2017 announced that the brolucizumab 6-mg group met the primary endpoint of non-inferiority compared to aflibercept every eight weeks (measured by mean change in BCVA from baseline to week 48). A majority of patients, 57 percent (HAWK) and 52 percent (HARRIER), were maintained exclusively on a 12-week interval immediately following the three-month loading phase through week 48. The press release didn’t specify the mean number of injections that patients received after their three loading doses, a key metric for comparison to current therapies and regimens. The rate of ocular and non-ocular adverse events were comparable between aflibercept and brolucizumab.6
• Abicipar pegol. Abicipar pegol (Allergan and Molecular Partners) is a monoDARPin (Designed Ankyrin Repeat Protein) that blocks all isoforms of VEGF-A. The Phase IIb trial data suggested that it has more potent VEGF-A inhibition, smaller molecular size (34 kDa), and longer duration (12 weeks) than the currently available anti-VEGF-A agents. In a 20-week study, Abicipar 2 mg given every four weeks for three loading doses followed by monthly sham outperformed ranibizumab 0.5 mg given every four weeks. At the 20-week follow-up, the abicipar group gained a mean of nine letters, while the ranibizumab group gained only 4.7 letters with continued monthly dosing.7 (It’s worth noting that the ranibizumab group underperformed compared to previous major trials involving ranibizumab, such as the MARINA, CATT and ANCHOR trials, in which a mean of just over six, 6.5 and 10 letters were gained at 20-week follow-up, respectively.8-10) Quarterly dosing of ranibizumab 0.5 mg was tested in the EXCITE and PIER trials, and visual gains weren’t as favorable as those obtained in treatment arms of monthly ranibizumab treatment, although 18 percent and 13 percent of patients respectively gained ≥15 ETDRS letters on this regimen.11,12
The abicipar Phase III trials (NCT02462486) will compare treatment arms of abicipar every eight weeks (duration equal to aflibercept), abicipar every 12 weeks (duration superior to aflibercept), and ranibizumab every four weeks.13 In May of 2017, Allergan announced completion of patient recruitment in these two global AMD Phase III studies. If quarterly dosing of abicipar is found to be non-inferior to monthly ranibizumab and bimonthly aflibercept, this would represent a major step forward in reducing treatment burden.
In the Phase IIb study, approximately 10 percent of patients who received abicipar experienced episodes of ocular inflammation.14 Allergan has worked to improve the abicipar formulation and purification process with the goal of reducing the incidence of inflammation in Phase III trials. Similar problems with inflammation were encountered with ranibizumab during early clinical trials, which were successfully resolved in later trials. The retina community eagerly looks forward to more clinical trial information, especially the mean number of injections per year, in order to compare abicipar to the non-fixed dosing regimens of our currently used agents.
 |
Sustained Delivery Treatments
The need for frequent intravitreal injections has spurred several companies to develop sustained-release anti-VEGF-A devices.
• Ranibizumab Port Delivery System. The ranibizumab Port Delivery System (PDS), a nonbiodegradable port fixed to the sclera, has a 0.05-ml reservoir that can be refilled in the office. Phase I results indicated that it improved visual acuity comparable to monthly ranibizumab injections as reported in the MARINA and ANCHOR studies. The 20 patients in the trial gained a mean of 10 ETDRS letters and had received a mean of 4.8 refills by the 12-month follow-up. Three serious adverse events (related to the implantation procedure) were reported, including endophthalmitis and persistent vitreous hemorrhage.
In 2015, Genentech initiated a Phase II study, the LADDER trial (Long Acting Delivery of Ranibizumab, NCT02510794), to test the safety and efficacy of the port delivery system on a larger scale, with a focus on optimizing the treatment dose and refill intervals.15
• GB-102. GB-102 (GrayBug Vision) is sunitinib maleate, a multitargeted tyrosine kinase inhibitor with activity against both VEGF-A and platelet derived growth factor. It’s an injectable depot designed for twice- per-year formulation. The drug is encapsulated within bioerodible polymer nanoparticles that slowly degrade over time. The GrayBug formulation is designed to avoid the inflammatory response seen with other nanoparticles, and the nanoparticles remain at the injection site to avoid clouding the visual axis. In a rabbit laser CNV model, GB-102 was found to be superior to aflibercept, according to data published on the GrayBug website. Aflibercept is maximally effective when given at the same time as the laser injury, whereas GB-102 demonstrated equal efficacy when given 10 weeks prior to it. Phase I studies are in development.16
Topical Treatments
Another method for reducing the treatment burden is to eliminate the need for an injection, a path taken by the following investigational therapies:
• PAN-90806. PAN-90806 (PanOptica) is a VEGF-A inhibitor eye drop that has been shown to produce an anti-VEGF-A response comparable to currently available anti-VEGF-A therapies in half of 50 treatment-naïve nAMD patients, according to a Phase I/II study (NCT02022540). According to a presentation from the 2016 meeting of the Ophthalmic Innovation Summit, PanOptica planned to initiate a Phase I/II clinical trial of an updated formulation for patients with nAMD in late 2017 or early 2018. This newer suspension was designed to reduce the incidence of punctate keratopathy as a side effect.17
• LHA510. Alcon has developed a topical anti-VEGF-A medication, LHA510, that’s been tested as maintenance therapy for patients with nAMD. The Phase II study (NCT02355028) compares initial ranibizumab plus topical LHA510 for 84 days versus initial ranibizumab plus placebo, with the primary endpoint being number of patients requiring rescue ranibizumab injections before day 84.17
Oral Anti-VEGF Therapy: X-82
X-82 (Tyrogenex) is an oral anti-PDGF and VEGF-A inhibitor. In the Phase I dose-escalation study (NCT02348359), 10 of 35 patients (29 percent) didn’t complete the 24-week endpoint, with six (17 percent) withdrawing due to adverse events. The most common adverse events were diarrhea (n=6), nausea (n=5), fatigue (n=5) and elevated transaminase enzymes (n=4) that reversed with cessation of X-82.
In terms of efficacy, 24 of the 25 patients that completed the 24-week trial maintained or improved their visual acuity (mean +3.8 letters), and 15 of 25 (60 percent) did so without the need for rescue ranibizumab injections (as specified by predefined retreatment criteria). Mean central subfield thickness was reduced by a mean of 50 µm, and eight patients (all receiving at least 100 mg daily) maintained sustained reductions without the need for rescue ranibizumab injection.18
The Phase II APEX study (NCT02348359) is comparing X-82 with as-needed aflibercept injections to aflibercept monotherapy in patients with AMD.
Gene Therapy
Recently, the eye has become a target for investigational gene therapy due to the monogenic nature of many inherited retinal diseases, its accessibility, the tight blood-ocular barrier, the ability to non-invasively monitor for functional and anatomic outcomes, and its relatively immune-privileged state. Gene therapy for nAMD offers the promise of long-term continuous expression of anti-VEGF protein with a single administration. Viral vectors are conduits for transferring desired genetic information to host cells.
Vectors currently used in ocular gene therapy clinical trials include adeno-associated virus (small single-stranded DNA viruses of the parvovirus family) and lentivirus (RNA viruses of the retrovirus family). Both can transduce non-dividing cells, but AAV are non-integrating, while lentivirus integrate their genome into the host cell genome and have larger transgene capacity (approximately 10 kb vs 4.5-5.0 kb for AAV). After successful transduction of the genome, the target cells transcribe and translate the viral genetic material into therapeutic protein, which then modulates the pathogenesis of the targeted disease process.
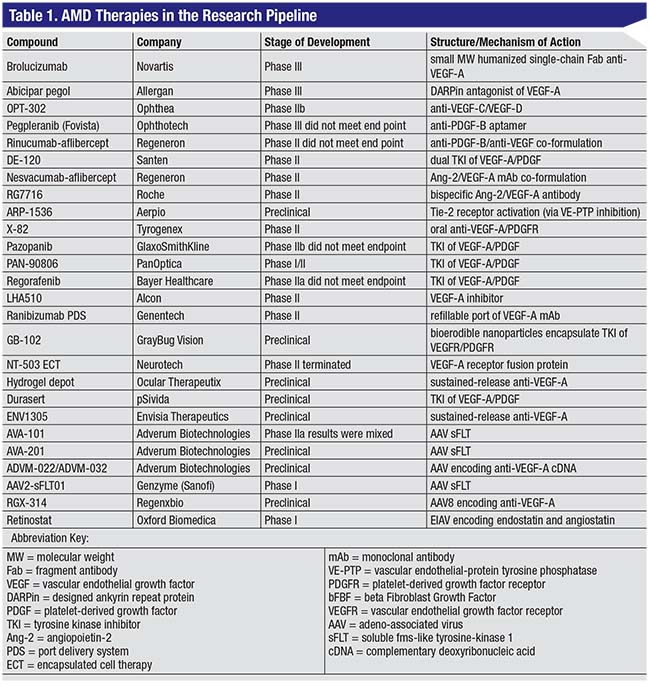
Gene therapy for AMD is still in its early phases of development and has not yet translated to consistently meaningful visual gains in AMD patients, but nonetheless represents an exciting area for future research.19 Gene therapies for AMD are summarized in Table 1.
When we take stock in the current state of ophthalmology, there are many experimental anti-VEGF agents and drug delivery systems under development that aim to decrease the treatment burden associated with frequent intravitreal injections. If one or more of these becomes a successful part of our everyday practice, treatment paradigms could be dramatically disrupted, improving the lives of an expanding population of seniors with AMD.
Dr. Hussain is a vitreoretinal surgery fellow at Bascom Palmer Eye Institute. Dr. Ciulla is the past co-director of the retina service and ocular angiogenesis research laboratory at Indiana University School of Medicine, and remains a volunteer clinical professor of ophthalmology. He serves on the board of directors of Midwest Eye Institute and is currently the medical strategy lead in ophthalmology at Spark Therapeutics. Neither Dr. Hussain nor Dr. Ciulla have financial interests in anti-VEGF therapies. REVIEW
1. JUSUFBEGOVIC D, MUGAVIN MO, SCHAAL S. EVOLUTION OF CONTROLLING DIABETIC RETINOPATHY: CHANGING TRENDS IN THE MANAGEMENT OF DIABETIC MACULAR EDEMA AT A SINGLE INSTITUTION OVER THE PAST DECADE. RETINA 2015;35:929-934.
2. SMITH AG, KAISER PK. EMERGING TREATMENTS FOR WET AGE-RELATED MACULAR DEGENERATION. EXPERT OPIN EMERG DRUGS 2014;19:157-164.
3. HOLEKAMP NM, LIU Y, YEH WS, ET AL. CLINICAL UTILIZATION OF ANTI-VEGF AGENTS AND DISEASE MONITORING IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. AM J OPHTHALMOL 2014;157:825-833 E821.
4. HUSSAIN RM, HARIPRASAD SM, CIULLA TA. TREATMENT BURDEN IN NEOVASCULAR AMD: VISUAL ACUITY OUTCOMES ARE ASSOCIATED WITH ANTI-VEGF INJECTION FREQUENCY. OPHTHALMIC SURG LASERS IMAGING RETINA 2017;48:780-784.
5. DUGEL P, WARBURTON J, WEICHSELBERGER A, SALLSTIG P. A 2-YEAR STUDY COMPARING THE EFFICACY AND SAFETY OF BROLUCIZUMAB VS AFLIBERCEPT IN SUBJECTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: TESTING AN ALTERNATIVE TREATMENT REGIMEN. PRESENTED AT: ASSOCIATION FOR RESEARCH IN VISION AND OPHTHALMOLOGY ANNUAL MEETING; 2016 MAY 1-5; SEATTLE, WA.
6. NOVARTIS RTH258 (BROLUCIZUMAB) DEMONSTRATES ROBUST VISUAL GAINS IN NAMD PATIENTS WITH A MAJORITY ON A 12-WEEK INJECTION INTERVAL. AVAILABLE AT: HTTPS://WWW.NOVARTIS.COM/NEWS/MEDIA-RELEASES/NOVARTIS-RTH258-BROLUCIZUMAB-DEMONSTRATES-ROBUST-VISUAL-GAINS-NAMD-PATIENTS. ACCESSED 12 FEBRUARY 2018.
7. ABICIPAR PEGOL (ANTI-VEGF DARPIN) AND BIMATOPROST SUSTAINED-RELEASE IMPLANT FOR GLAUCOMA. AVAILABLE AT: HTTPS://WWW.ALLERGAN.COM/MISCELLANEOUS-PAGES/ALLERGAN-PDF-FILES/RD_INVESTOR_PRESENTATION_JUN30_2014. ACCESSED 12 FEBRUARY 2018.
8. ROSENFELD PJ, BROWN DM, HEIER JS, ET AL. RANIBIZUMAB FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. N ENGL J MED 2006;355:1419-1431.
9. CATT RESEARCH GROUP, MARTIN DF, MAGUIRE MG, ET AL. RANIBIZUMAB AND BEVACIZUMAB FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. N ENGL J MED 2011;364:1897-1908.
10. BROWN DM, KAISER PK, MICHELS M, ET AL. RANIBIZUMAB VERSUS VERTEPORFIN FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. N ENGL J MED 2006;355:1432-1444.
11. SCHMIDT-ERFURTH U, ELDEM B, GUYMER R, ET AL. EFFICACY AND SAFETY OF MONTHLY VERSUS QUARTERLY RANIBIZUMAB TREATMENT IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: THE EXCITE STUDY. OPHTHALMOLOGY 2011;118:831-839.
12. REGILLO CD, BROWN DM, ABRAHAM P, ET AL. RANDOMIZED, DOUBLE-MASKED, SHAM-CONTROLLED TRIAL OF RANIBIZUMAB FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: PIER STUDY YEAR 1. AM J OPHTHALMOL 2008;145:239-248.
13. SAFETY AND EFFICACY OF ABICIPAR PEGOL IN PATIENTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. AVAILABLE AT: HTTPS://CLINICALTRIALS.GOV/CT2/SHOW/NCT02462486. ACCESSED 12 FEBRUARY 2018.
14. POSITIVE TOP-LINE DATA REPORTED FROM PHASE II STUDY FOR DARPIN ABICIPAR PEGOL IN WET AMD. AVAILABLE AT: HTTPS://WWW.MOLECULARPARTNERS.COM/POSITIVE-TOP-LINE-DATA-REPORTED-FROM-PHASE-2-STUDY-FOR-DARPIN-ABICIPAR-PEGOL-IN-WET-AMD/. ACCESSED 12 FEBRUARY 2018.
15. STUDY OF THE EFFICACY AND SAFETY OF THE RANIBIZUMAB PORT DELIVERY SYSTEM (RPDS) FOR SUSTAINED DELIVERY OF RANIBIZUMAB IN PARTICIPANTS WITH SUBFOVEAL NEOVASCULAR AGE-RELATED MACULAR DEGENERATION (AMD) (LADDER). AVAILABLE AT: HTTPS://CLINICALTRIALS.GOV/CT2/SHOW/NCT02510794. ACCESSED 31 JANUARY 2018.
16. GB-102 FOR TREATMENT OF NAMD. AVAILABLE AT: HTTP://WWW.GRAYBUG.COM/PIPELINE-TECHNOLOGY/GB-102/. ACCESSED 12 FEBRUARY 2018.
17. LHA510 PROOF-OF-CONCEPT STUDY AS A MAINTENANCE THERAPY FOR PATIENTS WITH WET AMD. AVAILABLE AT: HTTPS://CLINICALTRIALS.GOV/CT2/SHOW/NCT02355028. ACCESSED 12 FEBRUARY 2018.
18. JACKSON TL, BOYER D, BROWN DM, ET AL. ORAL TYROSINE KINASE INHIBITOR FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: A PHASE 1 DOSE-ESCALATION STUDY. JAMA OPHTHALMOL 2017;
135:7:761-767.
19. MOORE NA, BRACHA P, HUSSAIN RM, ET AL. GENE THERAPY FOR AGE-RELATED MACULAR DEGENERATION. EXPERT OPIN BIOL THER 2017;17:1235-1244.