Toxoplasmosis is the most common cause of infectious posterior uveitis worldwide, caused by an infection by the protozoan parasite Toxoplasma gondii. It’s found both in the United States and across the globe, and is an important consideration to rule out when evaluating any new posterior or panuveitis patient, especially when considering systemic or local (periocular or intraocular) steroid therapy.1,2,3 Here, we’ll review the finer points of diagnosis and treatment of the condition.
Background and Epidemiology
The high prevalence of T. gondii is made possible by its uniquely elaborate and elusive life cycle. In order to replicate, T. gondii cysts must be ingested by a felid (domestic or wild cat) and undergo sexual reproduction in their gastrointestinal tract with the help of specific digestive enzymes. Oocysts containing sporozoites are then excreted and once again ingested by a variety of hosts, where they undergo asexual reproduction into tachyzoites and finally bradyzoites, a low virulence form which can persist for long periods of time within tissues cysts until they are released and cause inflammation to adjacent tissues.3
Worldwide prevalence of toxoplasmosis infection is estimated to be 25 to 30 percent on average, but it varies heavily based on location and climate; national rates can range from ~10 to 20 percent in low-prevalence areas like North America, Southeast Asia and Northern Europe and as high as 80 percent in tropical environments like South America and tropical African countries.2,4
Toxoplasmosis can be acquired at any point of life through ingestion of oocysts or congenitally when a pregnant woman becomes infected and the parasites are passed through the placenta to the fetus.5 The latter is far less common and presents earlier in life with different patterns of chorioretinal scarring and inflammation that are discussed below.
 |
|
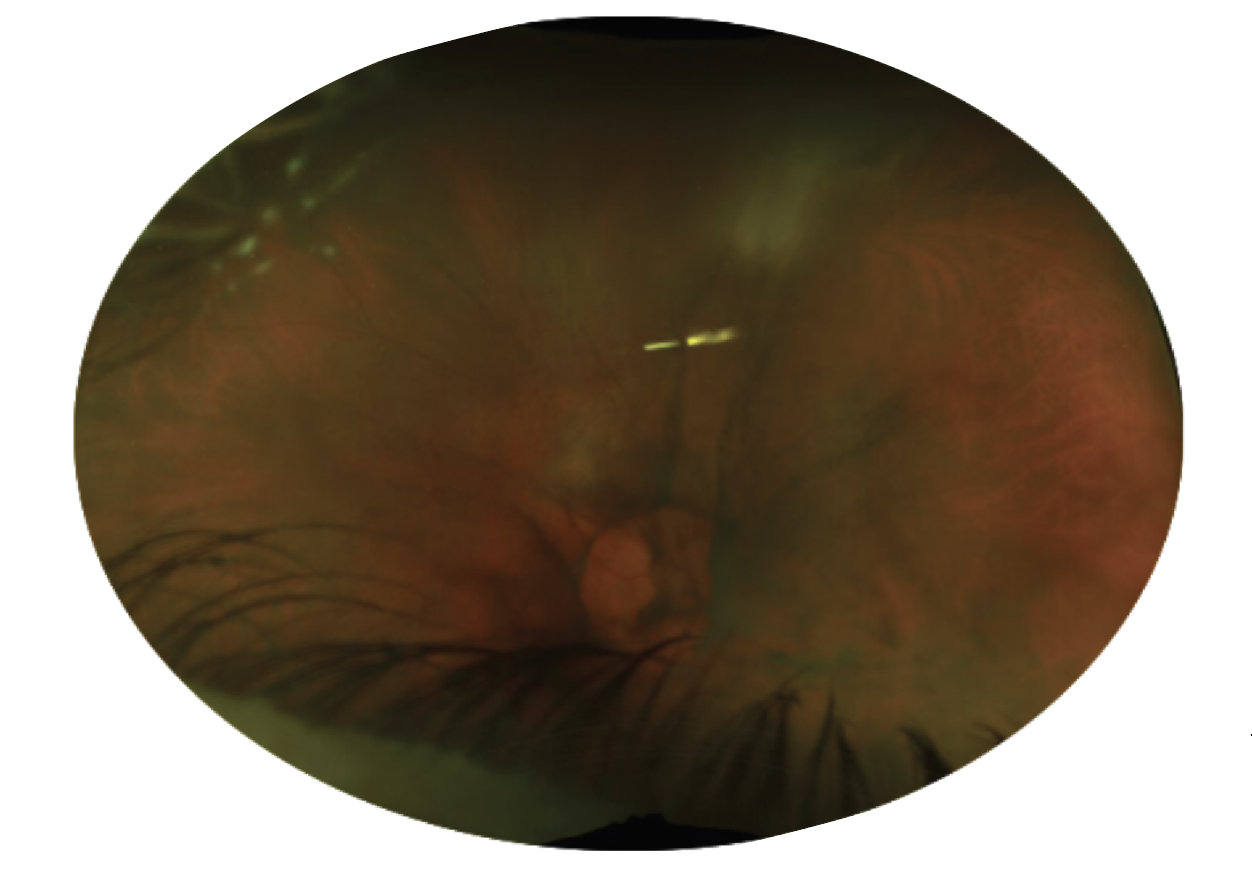
Figure 1. Ultra-widefield fundus imaging of the left eye of a 69-year-old patient with white retinitis seen superiorly through vitreous haze—the “headlight in the fog” appearance. This patient was treated topically for anterior uveitis alone for two years prior to presentation, with progressively worsening vitritis and deterioration in visual acuity. |
Clinical Presentation
Toxoplasmosis was first described as a congenital infection in 1923 and gained wider acknowledgement as a major cause of infectious posterior and panuveitis over the subsequent two decades.6,7 While there was initial skepticism over the role the parasite played in inflammatory eye disease, studies examining cadaver eyes as well as experimental non-human models helped to elaborate the role that the organism plays in causing retinochoroiditis in both immunocompetent and immunocompromised subjects.8,9,10
We now know that ocular toxoplasmosis can present with a variety of patterns and degrees of inflammation. Classically, patients will demonstrate an area of retinitis at the border of an inactive chorioretinal scar with some degree of vitritis and/or anterior inflammation.9 This has led to the typical description of a “headlight in the fog,” describing the appearance of the bright white, focal retinitis seen through vitreous haze (Figures 1 and 6).11,12 Vasculitis is often present adjacent to areas of retinitis and can show characteristic “Kyrieleis plaques” (Figure 2), which are segmental yellow-white lesions within retinal arteries. These plaques are also now referred to as segmental retinal arteritis due to unacceptable ideological beliefs of Werner Kyrieleis.13 Some studies have suggested a sex difference in patients, with female patients more likely to have multiple chorioretinal lesions as well as recurrent disease.14
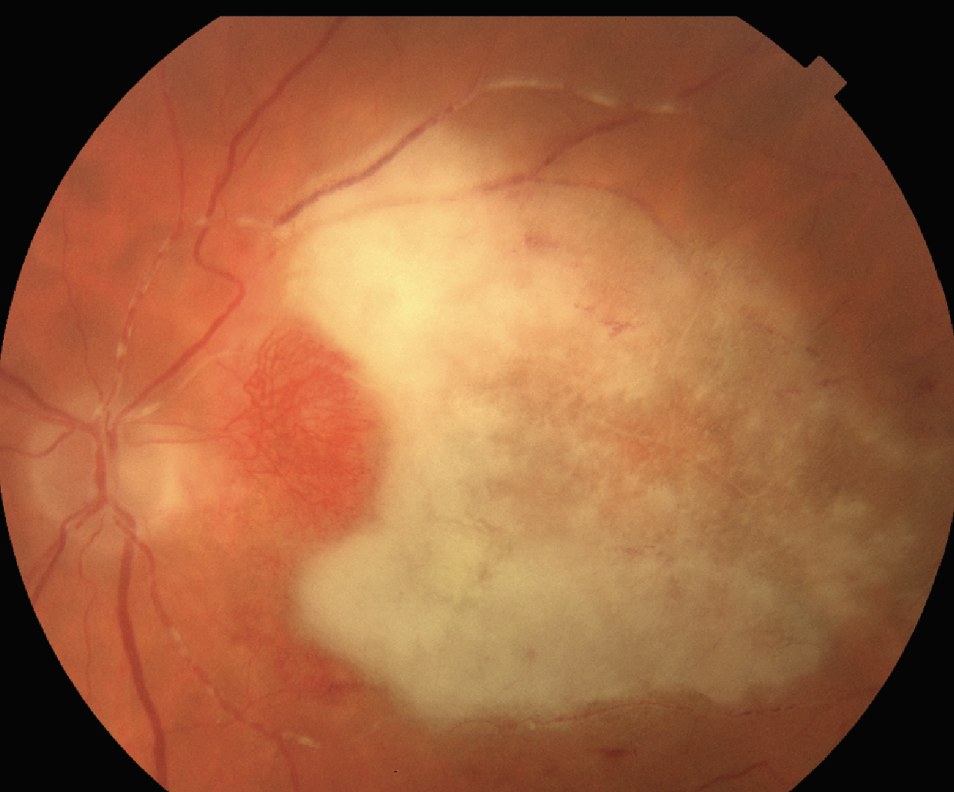 |
| Figure 2. Immunocompromised patient on systemic methotrexate who underwent posterior subtenon’s triamcinolone injection for initially presumed non-infectious uveitis. The vitreous was positive for toxoplasma with PCR testing. Color fundus photo demonstrating a large area of the macula affected by retinitis. Nasal collateralization is seen as well as Kyrieleis plaques, or segmental retinal arteritis, along the superior and inferior arcades. |
Atypical presentations may occur, especially in patients that are elderly or immunocompromised.15 In these patients, a high degree of suspicion must be maintained for all patients from isolated optic nerve edema, diffuse retinitis, retinal vasculitis and even scleritis. In these patients, it’s especially important to rule out or treat for infectious causes like toxoplasmosis, as they can have severe worsening with local or systemic steroid therapy without antimicrobial coverage.16,17,18 One atypical presentation includes atypical toxoplasmosis chorioretinitis mimicking acute retinal necrosis. Including toxoplasmosis PCR testing in initial aqueous taps can lead to timely diagnosis and avoid unnecessary antiviral local and systemic therapy.19
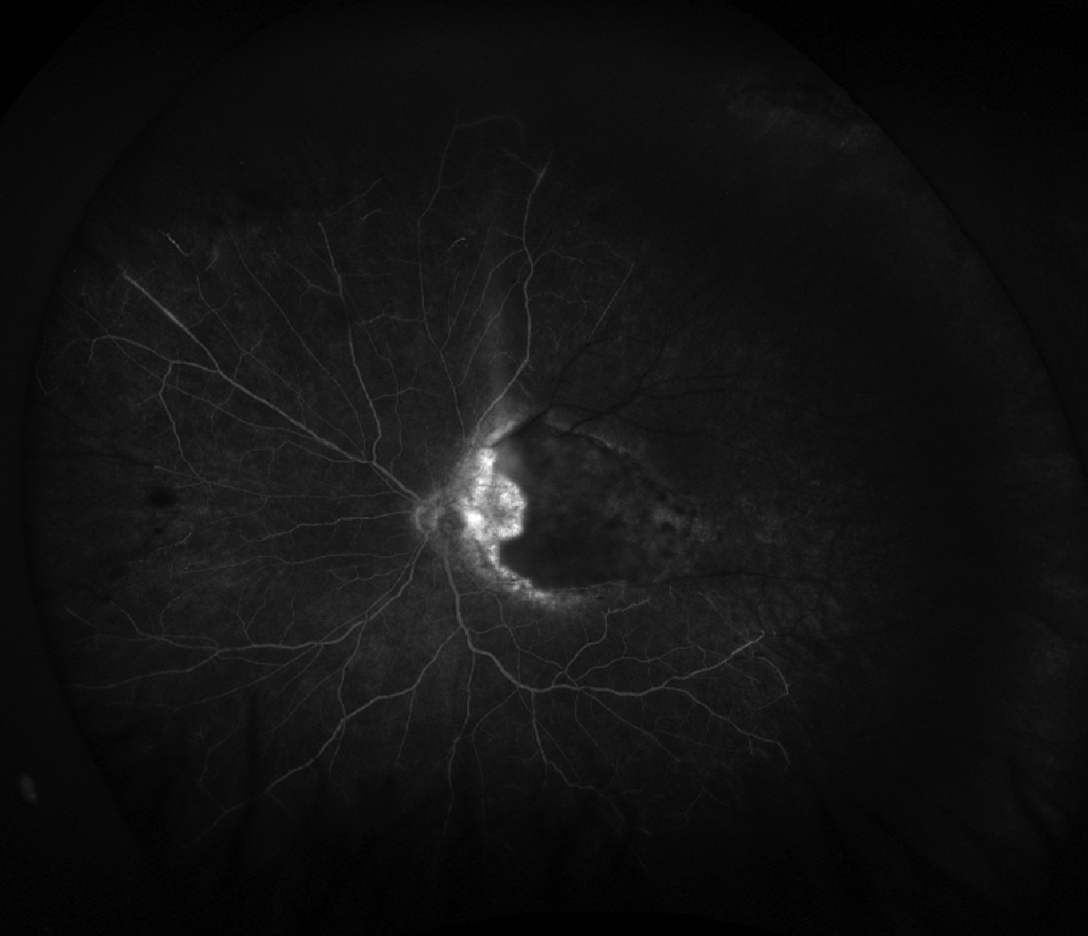 |
| Figure 3. Ultra-widefield fluorescein angiography of the patient in Figure 2 demonstrating early blockage of the active macular lesion as well as occlusion of superotemporal vessels. |
Choroidal neovascularization may rarely occur at or near previous sites of chorioretinal scarring and require intravitreal anti-VEGF treatment. This doesn’t usually present at the time of active inflammation. Similarly, late tractional and rhegmatogenous retinal detachments may occur from vitreous traction that develops as the inflammation improves. Surgical intervention may be needed to prevent permanent vision loss.
Congenital Toxoplasmosis
While patients with congenital toxoplasmosis and ocular involvement may not have any visual complications if scars are extramacular and they have no episodes of inflammation/retinitis, case series examining newborn babies screened for toxoplasmosis due to known maternal infection have shown high rates of ocular involvement at infancy.5 In one case series from Brazil, of 187 babies born to mothers with positive toxoplasma IgM, 29 were found to have congenital infections. Within this cohort, 19 (65.5 percent) were found to have ocular involvement.20
| Fun fact: Pyrimethamine, brand named Daraprim, was the source of public controversy when in 2015, Turing Pharmaceuticals and its founder Martin Shkrelli infamously bought the rights to the medication and increased its market price from $13.50 to $750/pill. This forced clinicians to change their practice patterns when treating ocular toxoplasmosis, as well as other conditions, and increased the need for data comparing the effectiveness of alternative therapies to the “gold standard.”34 |
Punctate outer retinal toxoplasmosis (PORT) is a unique clinical phenotype that presents more commonly in those with congenital toxoplasmosis and usually within the first two decades of life. These patients typically present with small, deep, hypopigmented retinal lesions that persist once the acute inflammatory phase has resolved. Vitreous inflammation is usually minimal in these patients, but retinitis is often accompanied by optic nerve involvement. Presentation can be similar to common non-infectious posterior uveitis, but can be differentiated using multimodal imaging and a high degree of suspicion.21,22,23
Patients with congenital toxoplasmosis are more likely to have bilateral involvement and more aggressive retinitis. They’re more likely to have recurrent episodes of inflammation and require long-term suppressive therapy, as discussed below.5
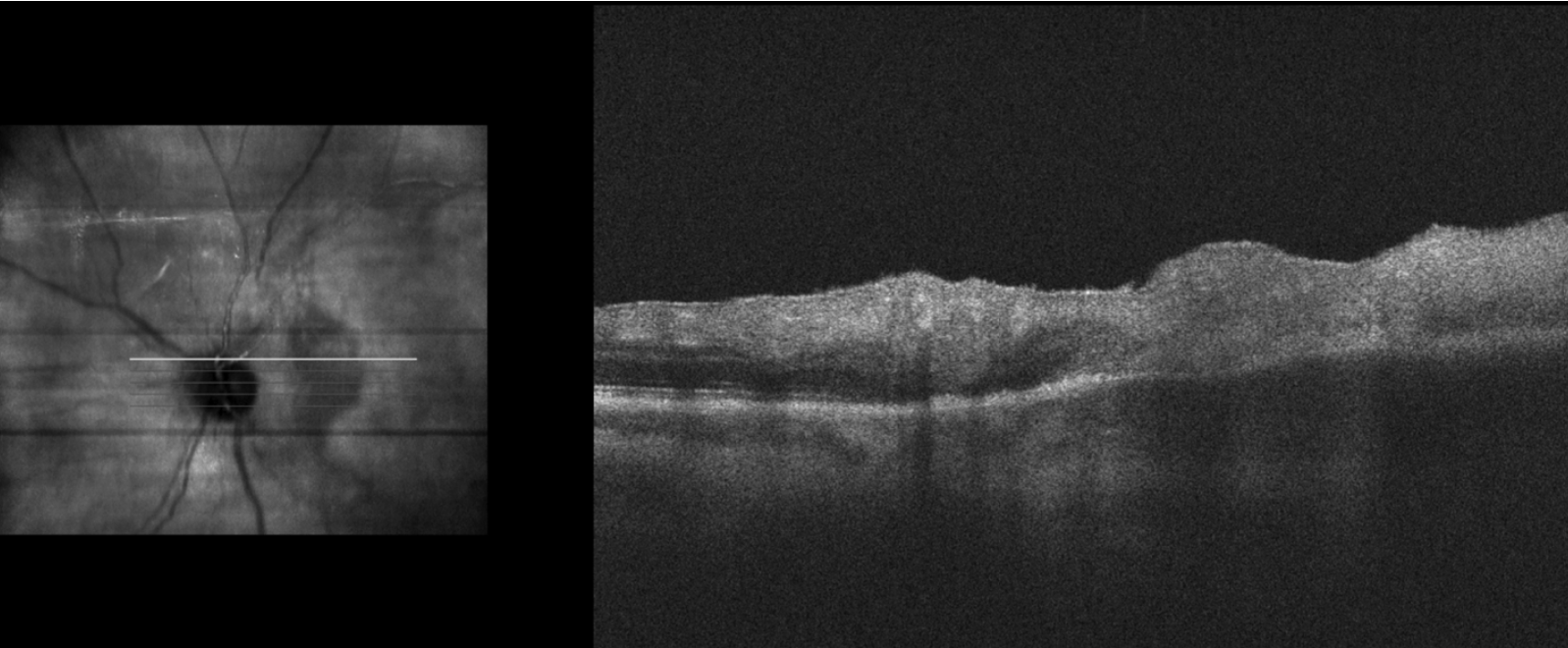 |
| Figure 4. Optical coherence tomography demonstrating full-thickness retinal hyper-reflectivity with loss of retinal laminations temporally, suggestive of retinitis. The choroid also appears to be thicker beneath this area although there’s signal blockage due to the overlying retinitis. |
Multimodal Imaging and Laboratory Testing
Ocular toxoplasmosis is a diagnosis made primarily based on clinical presentation. However, adjunctive testing can be useful to solidify the diagnosis and monitor for improvement.
Ultra-widefield color fundus imaging can provide objective yardsticks by which improvement in vitritis and retinitis can be observed. Fluorescein angiography can help differentiate active (leaking) and quiescent (staining without leaking) lesions as well as highlight areas of retinal vessel occlusion due to lesions (Figure 3).
Optical coherence tomography of the macula can assist in ruling-out the presence of cystoid macular edema and assist with the grading of vitreous cells. Raster scans through lesions can confirm the presence of retinitis and determine the depth of the lesion by looking for full-thickness hyper-reflectivity (Figure 4).10
Laboratory testing can be useful when the diagnosis of toxoplasmosis is unclear based on clinical presentation and imaging. PCR testing of aqueous or vitreous samples may be used with similarly high efficacy, though some studies have demonstrated relatively higher sensitivity in vitreous samples.24,25,26 Serological testing for T. gondii IgG and IgM may be used to evaluate prior or recent systemic infections and is mostly helpful for ruling out toxoplasmosis when IgG is negative.27
Management
In general, treatment is indicated for cases where vision is affected or threatened. This may be due to macula-involving retinitis, severe vitritis, optic neuropathy or cystoid macular edema. Inactive-appearing chorioretinal scarring attributed to prior infections can usually be monitored without treatment.28
Most cases of toxoplasmosis can be effectively managed with systemic medications that target the causative parasite and the accompanying inflammatory reaction. Prior to 2015, the gold standard for this treatment was “triple therapy” composed of pyrimethamine (100 mg for 2 days, then 25 mg daily), sulfadiazine (2 g) and folinic acid (5 mg). Pyramethamine’s effectiveness in this disease was originally described in 195628 and has been elaborated upon in the decades that followed.
Since then, regimens evaluating the utility of additional antimicrobials, specifically azithromycin, clindamycin, and trimethoprim-sulfamethoxazole (TMP-S), have been evaluated both in combination with pyrimethamine and as single-therapy agents. While all of these medications have demonstrated effectiveness across multiple studies, there have been scant prospective, randomized studies comparing one to another.31,32 In one study, Yanxia Zhang, MD, of China’s Sun Yat-sen University and co-authors used network meta-analysis to compare outcomes with systemic pyramethamine-sulfadiazine, clindamycin, azithromycin and TMP-S and found that clindamycin was associated with the greatest improvements in visual acuity and resolution of vitreous inflammation. The same study found that TMP-S was associated with lower rates of recurrence as well as side effects.33 We tend to use TMP-S as first-line treatment when no contraindications exist.
Dosages for treatment of active ocular toxoplasmosis are:
- pyrimethamine 75 to 100 mg loading dose, followed by 50 mg + sulfadiazine 1 to 4 g daily + folinic acid 5 mg;
- azithromycin 250 to 500 mg daily;
- clindamycin 300 mg four times daily; and
- trimethoprim-sulfamethoxazole 800-60 mg (Bactrim DS) twice daily
Importantly, pyrimethamine is a folic acid inhibitor and is associated with the highest risk of complications, including thrombocytopenia, leukopenia and fevers. Patients receiving pyrimethamine should also take folic acid supplementation and be monitored weekly with bloodwork. Patients receiving TMP-S should be screened and periodically monitored for kidney function testing.29,32
Intravitreal clindamycin (1 mg/0.1 ml) may be considered when there are contraindications to systemic therapy, such as in the first trimester of pregnancy, as well as for macular lesions where a faster response is desired. This may be used alone or in conjunction with systemic and/or intravitreal steroids.35,36
The role of systemic steroids is generally to control robust inflammatory reactions associated with toxoplasma infections, usually severe vitritis. Oral prednisone may be started up to 1 mg/kg depending on the degree of inflammation and titrated slowly over weeks-to-months until the vitritis is resolved and retinitis appears inactive. Figures 1 and 4 show the same patient who demonstrates an impressive response after five months of systemic TMP-S and prednisone. The prednisone was slowly tapered on a monthly basis as the patient’s vitritis improved. Intravitreal dexamethasone has also demonstrated good effectiveness when used in combination with intravitreal clindamycin.37, 38 Topical steroids and cycloplegics may be used adjunctively when anterior inflammation is present.
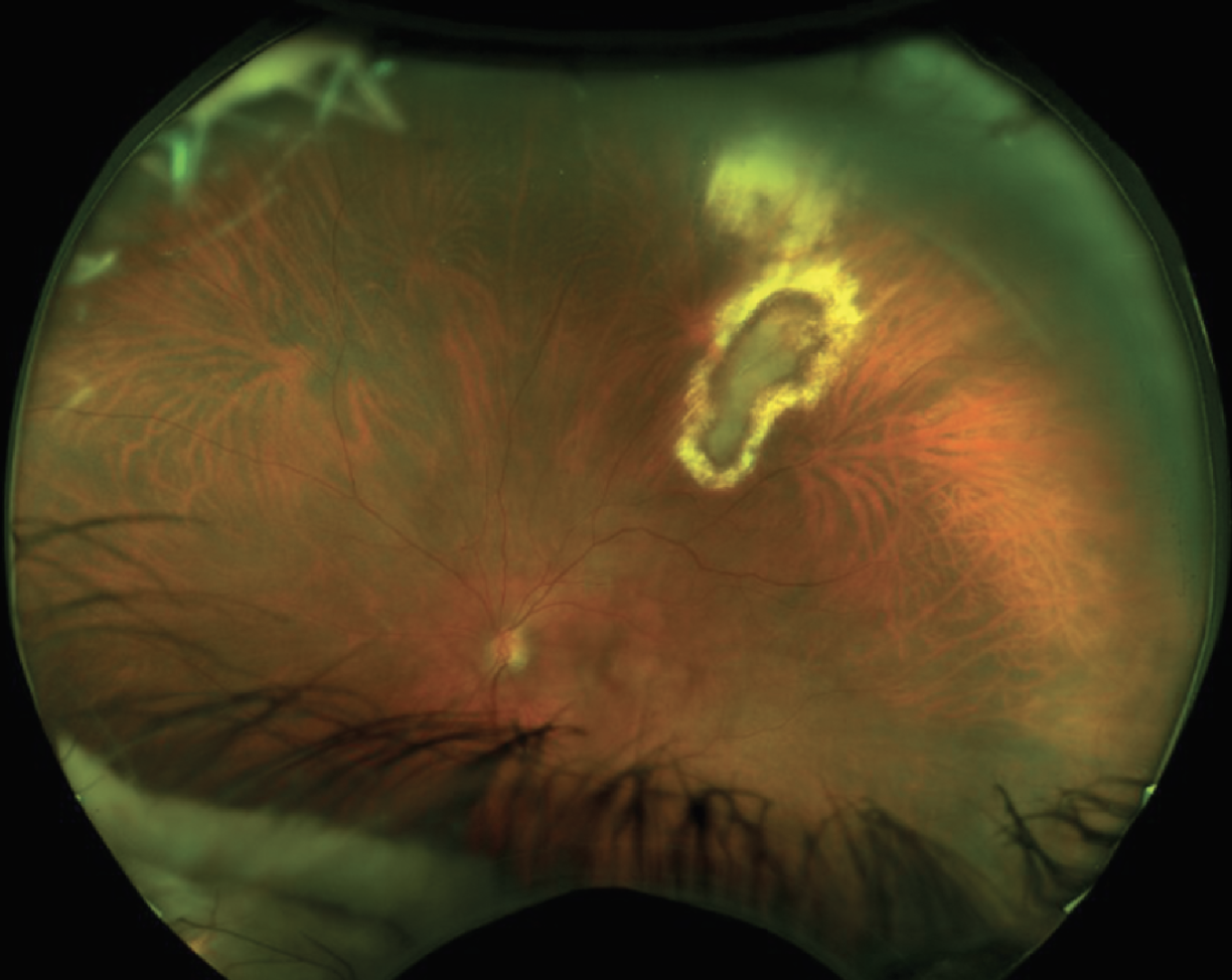 |
| Figure 5. The same patient in Figure 1 after five months of treatment with TMP-S and prednisone, which was slowly tapered. Visual acuity improved from 20/50 to 20/20 with resolution of vitritis. Active-appearing central retinitis and atrophic borders still remained. |
Prophylaxis
Recurrence of ocular inflammation isn’t uncommon and has been estimated to occur in 5 to 15 percent of patients within two years of initial treatment and increases with years of follow-up. In many patients, once the active disease process has been controlled, consideration must be given as to whether patients should receive lower-dose prophylactic treatment with systemic therapy for a year or more. This is aimed at preventing recurrence in high-risk patients and preventing systemic spread in immunocompromised patients. A randomized control study compared a year of treatment with TMP-S three times a week to placebo and found a dramatically lower probability of recurrence in the TMP-S group (1.39 percent) compared to the placebo group (27.34 percent) after six years of follow-up.31 The pros and cons of continued TMP-S treatment after the active infection is healed versus observation should be discussed with the patient. Given the strong data on the reduced risk of recurrence with continued use for at least one year after treatment, we encourage its use, especially in recurrent cases. However, the patient’s decision after understanding the risks and benefits is of course the final determining factor.
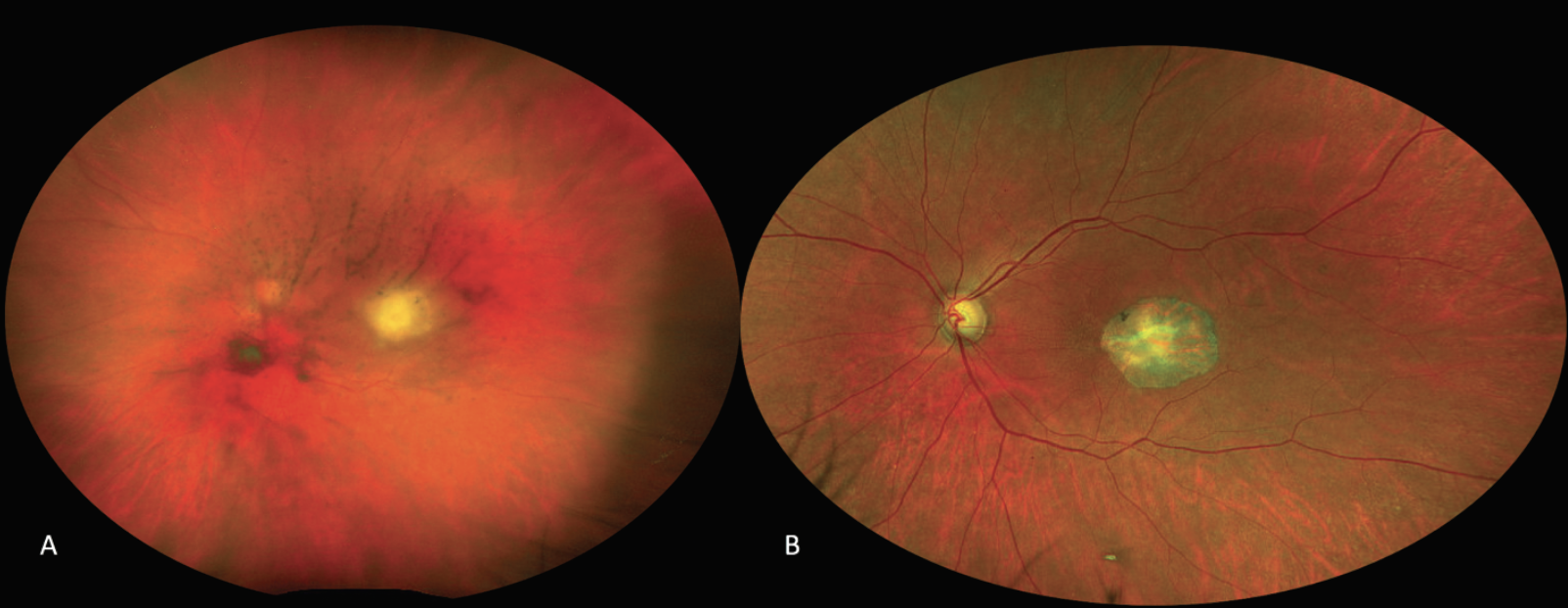 |
| Figure 6. Ultra-widefield fundus imaging of a 63-year-old woman who presented with floaters and blurry vision, whose exam demonstrated vitreous haze with a white macular retinitis lesion, a nice example of the classic “headlight in the fog” appearance (A). She was treated with oral TMP-S, prednisone, and intravitreal clindamycin with a resolution of the vitritis, pigmentation of the lesion, and a best-corrected visual acuity of 20/20 (B). |
In conclusion, toxoplasmosis retinochoroiditis is the most common cause of infectious posterior uveitis worldwide and can lead to varying degrees of vision loss due to macular or optic nerve involvement, vitritis and cystoid macular edema. Treatment includes systemic and/or intravitreal antimicrobials targeting the underlying organism T. gondii, as well as steroids in some cases to control the accompanying inflammatory reaction, and should be titrated over weeks-to-months until resolution of all active inflammation is observed. Multimodal imaging techniques and laboratory testing of serum as well as ocular fluids can assist with diagnosis when the picture is unclear, and prophylactic antimicrobials can be considered for patients at high risk for recurrence.
Dr. Fell is a first-year retina fellow at Tufts/Ophthalmic Consultants of Boston.
Dr. Mammo is a vitreoretinal surgical and uveitis staff physician at the Cleveland Clinic and an assistant professor of ophthalmology at the Cleveland Clinic Lerner College of Medicine/Case Western Reserve University.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
1. https://www.cdc.gov/parasites/toxoplasmosis/
2. Holland GN. Ocular toxoplasmosis: A global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003;136:6:973-88.
3. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012;25:2:264-96. Erratum in: Clin Microbiol Rev 2012;25;3:583.
4. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965–1976
5. Kieffer F, Wallon M. Congenital toxoplasmosis. Handb Clin Neurol 2013;112:1099-101.
6. Jankû J. Pathogenesa a Pathologická Anatomie T. Zv. Vrozeneho Kolobomu Žlute Śkurny Oku Normál ně Velikém a Mikrophtalmickěm s Nǎlezem Parasitu v Sitnici. Cǎs Lék Čes 1923;62:1021-1027
7. Wolf A, Cowen D, Paige B. Human toxoplasmosis: Occurrence in infants as an encephalomyelitis verification by transmission to animals. Science 1939;10:89:2306:226-7.
8. Holland GN, Engstrom RE Jr, Glasgow BJ et al. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol 1988;106:6:653–667
9. Holland GN, O'Connor GR, Diaz RF, Minasi P, Wara WM. Ocular toxoplasmosis in immunosuppressed nonhuman primates. Invest Ophthalmol Vis Sci 1988;29:6:835-42.
10. Kalogeropoulos D, Sakkas H, Mohammed B, Vartholomatos G, Malamos K, Sreekantam S, Kanavaros P, Kalogeropoulos C. Ocular toxoplasmosis: A review of the current diagnostic and therapeutic approaches. Int Ophthalmol 2022;42:1:295-321.
11. Atmaca LS, Simsek T, Batioglu F. Clinical features and prognosis in ocular toxoplasmosis. Jpn J Ophthalmol 2004;48:4:386-91.
12. Da Mata AP, Orifice F. Toxoplasmosis. In: Foster CF, Vitale AT, eds. Diagnosis and Treatment of Uveitis. Philadelphia: Saunders, 2002.
13. Gologorsky D. Eponymous dishonor: Kyrieleis plaques. Retina 2018;38:7:1261-2
14. Lyons MR, Arantes T, Vieira BR, et al. Impact of gender on clinical features and outcomes of ocular toxoplasmosis. British Journal of Ophthalmology. May 24, 2023. (Epub ahead of print.) doi: 10.1136/bjo-2023-323227.
15. Miserocchi E, Modorati G, Rama P. Atypical toxoplasmosis masquerading late occurrence of typical findings. Eur J Ophthalmol 2009;19:6:1091–1093.
16. Fish RH, Hoskins JC, Kline LB. Toxoplasmosis neuroretinitis. Ophthalmology 1993;100:8:1177–1182.
17. Jensen E. Retino-chorioiditis juxtapapillaris. Arch F Ophth 1908;69:41.
18. Rudich DS, Bhatnagar P. Scleritis associated with toxoplasmic retinochoroiditis. Arch Ophthalmol 2012;130:5:659–660 58.
19. Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology 2004;111:4:716-725.
20. Gilbert RE, Freeman K, Lago EG, et al. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis.2008;2(8):e277.
21. de Souza EC, Casella AM. Clinical and tomographic features of macular punctate outer retinal toxoplasmosis. Arch Ophthalmol 2009;127:10:1390–1394.
22. Doft BH, Gass DM. Punctate outer retinal toxoplasmosis. Arch Ophthalmol 1985;103:9:1332–1336.
23. Yannuzzi NA, Gal-Or O, Motulsky E et al. Multimodal imaging of punctate outer retinal toxoplasmosis. Ophthalmic Surg Lasers Imaging Retina 2019;50:5:281–287.
24. Farhadi A, Haniloo A, Fazaeli A, Moradian S, Farhadi M. PCR-based Diagnosis of Toxoplasma Parasite in Ocular Infections Having Clinical Indications of Toxoplasmosis. Iran J Parasitol 2017;12:1:56-62.
25. Okhravi N, Jones CD, Carroll N, Adamson P, Luthert P, Lightman S. Use of PCR to diagnose Toxoplasma gondii chorioretinitis in eyes with severe vitritis. Clin Exp Ophthalmol 2005;33:2:184-7.
26. Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002;134:1:102–14.
27. Roh M, Yasa C, Cho H, et al. The role of serological titres in the diagnosis of ocular toxoplasmosis. Acta Ophthalmol 2016;94:5:521–522.
28. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: Clinical features and prognosis of 154 patients. Ophthalmology 2002;109:5:869-78.
29. Perkins ES, Schofield PB, Smith CH. Treatment of uveitis with pyrimethamine (daraprim). Br J Ophthalmol 1956;40:10:577-86.
30. Lam S, Tessler HH. Quadruple therapy for ocular toxoplasmosis. Can J Ophthalmol 1993;28:2:58-61.
31. Fernandes Felix JP, Cavalcanti Lira RP, Grupenmacher AT, Assis Filho HLG, Cosimo AB, Nascimento MA, Leite Arieta CE. Long-term results of trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrent Toxoplasma gondii retinochoroiditis. Am J Ophthalmol 2020;213:195-202.
32. Soheilian M, Sadoughi MM, Ghajarnia M, et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005;112:1876-82.
33. Zhang Y, Lin X, Lu F. Current treatment of ocular toxoplasmosis in immunocompetent patients: A network meta-analysis. Acta Tropica 2018;185:52–62.
34. Pollack A. Drug goes from $13.50 a tablet to $740, overnight. New York Times. September 20, 2015.
35. Kump LI, Androudi SN, Foster CS. Ocular toxoplasmosis in pregnancy. Clin Exp Ophthalmol 2005;33:5:455–60.
36. Rothova A, Bosch-Driessen LEH, van Loon NH, et al. Azithromycin for ocular toxoplasmosis. British Journal of Ophthalmology 1998;82:1306-1308.
37. Lasave AF, Díaz-Llopis M, Muccioli C, Belfort R Jr, Arevalo JF. Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology 2010;117:9:1831-8.
38. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 2011;118;1:134-41.



