 |
|
(Image courtesy of Dr. Maturi and colleagues.) |
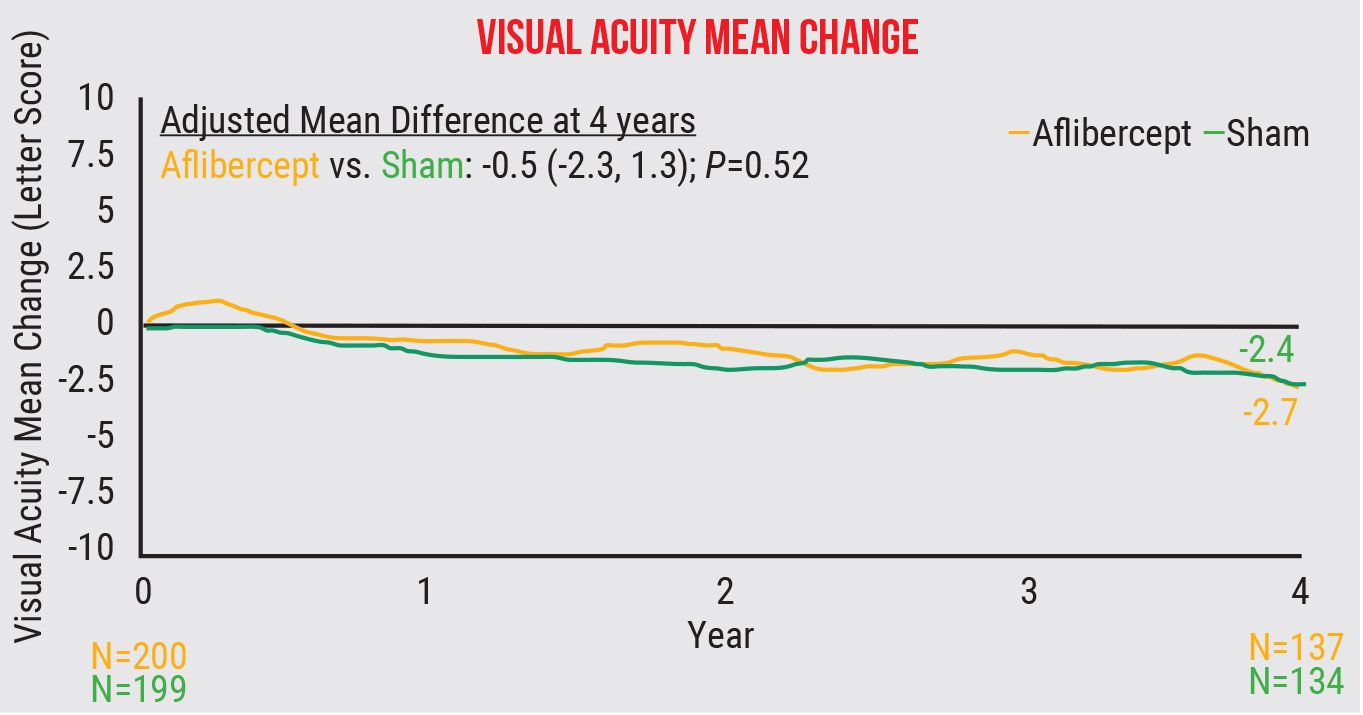
The four-year findings from the NEI-supported Protocol W clinical trial show that early treatment of non-proliferative diabetic retinopathy with anti-VEGF agents confers no visual acuity benefit in the long term.1
Protocol chair Raj Maturi, MD, of Indiana University School of Medicine and Retina Partners Midwest, explains that “monitoring patients for signs of disease progression, such as vision loss, retinal edema and neovascularization, and treating only as needed once complications develop is the best course of action.”
In the study, 328 patients (399 eyes) were randomized to 2-mg aflibercept injections or sham injections. During the first two years, eight injections were administered at one month, two months, four months and every four months. Injections were then continued quarterly through year four unless the eye improved to mild disease. Eyes in either group that developed vision-threatening complications were treated with additional anti-VEGF as necessary.
The cumulative probability of developing proliferative diabetic retinopathy or center-involved diabetic macular edema was 33.9 percent in the treatment group and 56.9 percent in the sham group (p<0.001). The visual acuity change from baseline to four years wasn’t significant, with a mean change of -2.7 ±6.5 letters in the treatment group and -2.4 ±5.8 letters in the sham group (p=0.52).
At the start of the study, the researchers had hypothesized that early injections would have a protective effect on moderate and severe non-proliferative diabetic retinopathy, but the four-year results showed that almost one-third of eyes still developed complications despite early treatment and saw no significant functional vision gains.
“Treating prophylactically doesn’t cause significant harm, but we found that holding off on treatment until disease worsening resulted in a reduction in the average number of injections,” Dr. Maturi says. “There was an average of 13 injections in the early-treatment group and just 3.5 injections in the group that waited until worsening. This study demonstrated that there’s no difference in visual acuity whether we wait or not, but there is a substantial difference in treatment burden.
“By treating early, we increase the number of injections given to patients and therefore increase the patient burden, cost, and risk of injection related complications,” he continues. “We want to decrease this number. An average injection of aflibercept costs around $2,000. A difference of 10 injections, as we saw in our study, is almost $20,000 per patient, on average, over four years.
Does early treatment ever make sense for certain patients? “Prophylactic treatment will slow some disease progression, but it won’t change many of the aspects we worry about such as nonperfusion,” Dr. Maturi says. “Anti-VEGF treatment for diabetic retinopathy is a long-term commitment. Some patients may be okay with that and want a treatment that will keep the disease from progressing. That’s up to them. Many doctors feel that way too. But many of us also look for an endpoint, and one of those endpoints may be that we treat with injections only to prevent bleeding and vision loss. So, if we treat patients when their situation is more advanced, we also prevent it from happening, in a way. Many others, however, wish to decrease treatment burden for patients and now have very clear evidence that waiting until the disease progresses is not going to compromise their patient’s visual acuity.
“It may make sense to treat poorly compliant patients prophylactically but, unfortunately, the protection offered by early treatment goes away if the patient isn’t treated regularly,” he continues. “So, it’ll delay worsening but if the patient doesn’t return for follow-up, they’ll still progress to worsening retinopathy.”
Dr. Maturi adds that the compliance data in the study points to a need for improving loss-to-follow-up. “We worked hard to ensure patients came back in this study,” he says. “We had a research coordinator follow up with patients, reimbursed patients for their time and travel, and other things of that nature. Even with that, at the end of four years, we had a follow-up rate of only around 80 percent. So, 20 percent of patients—one in five—couldn’t come for follow-up in a study where we were really pushing them to come back. Looking at data from large datasets, we see that diabetic patients, unfortunately, will often miss those follow-up exams due to various socio-economic factors. Treating patients with more permanent solutions is an important area of development. We have to do more to minimize treatment burden so patients can maintain visual function.”
Ultimately, Dr. Maturi says that it’s crucial to monitor diabetic retinopathy carefully and treat patients as soon as they meet high-risk criteria for proliferative diabetic retinopathy as well as diabetic macular edema with vision loss—which is a two-line loss of vision with associated macular thickening. “Initiating therapy when patients reach those thresholds for diabetic macular edema and proliferative diabetic retinopathy is just as safe as treating early,” he says.
Dr. Maturi is a consultant for Allegro, Allergan, Allgenesis, Eli Lilly, Dutch Ophthalmic, Novartis, neurotech and Jaeb Center for Health Research. He receives research support from Allergan, Genentech, Ophthea, Kalvista, Samsung Bioepies, Graybug, Santen, Thromobgenics, Gyroscope, Gemini, Boehringer Ingelheim, Allegro, Senju, Ribomic, NGM biopharmaceuticals, Unity, Graybug and Clearside. He’s also the Safety Committee Chair of Aiviv.
1. Maturi RK, Glassman AR, Josic K, et al. Four-year visual outcomes in the Protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA 2023;329:5:376-385.
Apellis’ Syfovre Approved for Geographic Atrophy
Retina specialists will soon be able to treat patients with geographic atrophy in hopes of mitigating the loss of retinal cells and subsequent visual impairment that often develops in those with dry AMD. In February, the FDA approved the use of pegcetacoplan, a C3 inhibitor, from Apellis Pharmaceuticals for this indication. It will be marketed under the brand name Syfovre and will be priced at $2,190 per vial, the company announced.
In an investor call, Chief Medical Officer Caroline Baumal, MD, estimated that patients will need six to eight vials of Syfovre per year. A program called ApellisAssist will provide insurance support, financial assistance and disease education for eligible patients, the company said in the release.
Syfovre is approved for GA patients with or without subfoveal involvement and should be administered every 25 to 60 days, a flexible dosing schedule that should help patients and doctors alike maintain therapy over the long haul, the company noted.
Patients need to commit to an extended course of therapy to experience the drug’s benefits. “This isn’t a drug that flips the switch on day one,” Apellis CEO Cedric Francois, MD, PhD, told Fierce Pharma in a recent interview. “It’s a drug where you make an investment over many years, and where the longer you stick to the therapy, the better this drug is going to work for you.”
In its FDA clinical trials (OAKS and DERBY), the drug “reduced the rate of GA lesion growth compared to sham and demonstrated increasing treatment effects over time, with the greatest benefit (up to 36 percent reduction in lesion growth with monthly treatment in DERBY) occurring between months 18 and 24,” according to the press release. OAKS and DERBY subjects are currently participating in three-year extension trials to gauge the durability of treatment effect out to five years.
More than one million Americans have developed GA, Apellis says. The condition arises from the combined effects of genetic influencers, environmental stressors and the aging process. Central to the pathogenesis is the complement cascade, an immune response in which a protein compound called membrane attack complex is created, which then adheres to cells and causes lysis and cell death. Pegcetacoplan blocks the cleavage of complement protein C3 into C3a and C3b; the latter is an upstream precursor of membrane attack complex.
Apellis says that Syfovre availability will begin in March. For more information, visit https://syfovreecp.com.
Customized Cross-linking in Keratoconus
Upon evaluating the visual and tomographic results of customized crosslinking using excimer laser-assisted epithelium removal and topography-guided irradiation in the treatment of progressive keratoconus, researchers recently reported that one-year outcomes showed a significant improvement in visual acuity and stabilization of disease progression.
The prospective, non-randomized clinical trial enrolled 37 eyes of 32 patients with documented progressive keratoconus. Following de-epithelization with phototherapeutic keratectomy, customized UV irradiation was performed, designed as three concentric circular areas centered on the thinnest point. Energy exposure was 5.4 J/cm2 in the outer circle and then increased centripetally to 7.2 J/cm2 and 10 J/cm2. Corrected distance visual acuity, refractive outcomes and Scheimpflug tomography were assessed at baseline and six and 12 months postoperatively.
Mean diameter for treated areas was 6.17 mm, 4.45 mm and 2.58 mm for the outer, medium and inner circle, respectively. At the one-year follow-up, mean CDVA improved significantly from 0.38 logMAR to 0.20 logMAR, with 92 percent of eyes retaining or improving CDVA. Mean preoperative minimum pachymetry decreased from 449.26 µm to 443.26 µm. The maximal curvature (Kmax) decreased significantly from 58.50 D to 57.05 D. After one year, 92 percent of eyes showed no signs of progression.
“The possibility to have a treatment alternative that’s also capable of simultaneously improving the patient’s vision provides us with a new therapeutic option and can help reduce the need for combined procedures for keratoconus,” the study authors wrote in their paper.
1. Gil JQ, Rosa AM, Costa AE, et al. Customized corneal crosslinking with excimer laser assisted epithelium removal for progressive keratoconus—one year results. J Cataract Refract Surg. February 13, 2023. [Epub ahead of print].
Industry News Alcon Settles Femto Litigation with J&J Alcon entered into a settlement agreement with J&J Surgical Vision to resolve its pending legal proceedings relating to femtosecond laser-assisted cataract surgery devices. As part of the resolution, the parties exchanged cross-licenses of certain intellectual property and other mutually agreed upon covenants and releases, and Alcon will make a one-time payment to J&J Surgical Vision of $199 million for those rights and to resolve related worldwide intellectual property disputes.
FDA Accepts Ocuphire’s New Drug Application Ocuphire announced the FDA accepted the New Drug Application for Nyxol (phentolamine ophthalmic solution 0.75%) for the treatment of pharmacologically induced mydriasis.
Eylea Approved for ROP Regeneron Pharmaceuticals received FDA approval of Eylea (aflibercept) Injection to treat preterm infants with retinopathy of prematurity. |