The wet form of macular degeneration remains the leading cause of blindness in developed countries. In some patients, however, dry macular degeneration alone may cause moderate to severe vision loss. While a variety of new therapies for wet macular degeneration are under evaluation, with the important exception of vitamin supplements, there are no proven therapies for advanced dry macular degeneration. Recently, a therapy called rheopheresis blood filtration has been studied as a new treatment for those patients with advanced dry macular degeneration. Here we review the background and preliminary results of this controversial new therapy.
Methodology and Rationale
Rheopheresis is an apparently safe and effective form of membrane differential filtration for the elimination of high molecular weight proteins. This process may improve the flow of blood through small vessels in the body by reducing blood and plasma viscosities as well as erythrocyte and thrombocyte aggregation. Since impaired blood flow in vessels beneath the retina—in the choroid—is thought to contribute to the development of dry macular degeneration, rheopheresis was introduced as a possible treatment for this condition.
The rationale for the use of rheopheresis for the treatment of a variety of age-related disorders is based on the theory that age-related disorders such as macular degeneration are diseases of the microcirculation (aging) and are mediated by circulating macromolecules that directly and indirectly down-regulate microvascular function. The process of rheopheresis begins with a filter for plasma separation, which is followed by filtration of macromolecules from the plasma. A 25-nm microfilter isolates the macromolecules in the ultrafiltrate and smaller molecules return in the circulation. Filtered macromolecules and their functions include: a2-macroglobulin, a primary mediator of plasma viscosity; fibrinogen, a mediator of thrombosis; the LDL-cholesterol complex, a primary mediator of atherogenesis; and immune complexes, particularly with IgA, which modulate expression of a variety of cell surface receptors.
Clinical Studies: Trials and Outcomes
Rheopheresis has been used in Europe for a number of years as a treatment for a variety of age-related disorders. While a number of retrospective studies have been published, there is a paucity of prospective randomized, controlled clinical studies. Therapeutic plasma exchange, a Food and Drug Administration-approved technology for the treatment of numerous conditions, created a wave of controversy when it was introduced in the United States as an unproven treatment for AMD. Given the potential benefits for patients with macular degeneration, several physicians introduced the treatment in the United States.
Empirically, they observed that a subset of patients in whom significant debris was found in the filters seemed to benefit the most from the treatment. Specific macromolecules were identified (see above) and then a pilot study was conducted at the University of Utah, Salt Lake City, enrolling patients with elevated levels of these specific molecules with encouraging results. A larger study was then proposed and is now underway—the Multicenter Investigation of Rheopheresis for AMD. The MIRA-1 study is a randomized, placebo-controlled clinical trial evaluating the use of rheopheresis in patients with large semi-soft drusen, decreased visual acuity and elevated plasma levels of two out of three high-risk markers: fibrinogen, cholesterol or IgA. This 12-month study is now under way with plans to enroll 180 intent-to-treat patients with large soft drusen, ETDRS visual acuity in the range of 20/32 to 20/125 and elevated levels of two of three rheologic markers: total cholesterol, IgA and fibrinogen.
 |
There will be up to 12 sites participating. Patients are all on high dose antioxidant vitamins with zinc, along the guidelines of the formulation used in AREDS, the Age-Related Eye Disease Study. The treatment protocol randomized 2:1 (treat: placebo) provides eight pulsed treatments over a 10-week period at the start of the year. Patients are followed for one year and assessed for the primary endpoint of best corrected visual acuity.
Visual Acuity
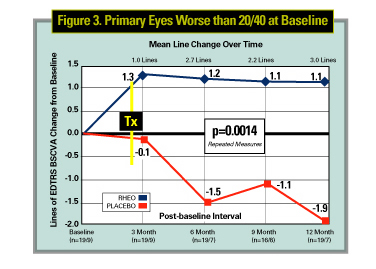
The initial results from the MIRA-1 trial for the first 43 patients completing the first year of the study were reported at the 2002 American Academy of Ophthalmology meeting. In those patients with a best corrected vision worse than 20/40, there was a 1.1-line (ETDRS) improvement of vision over the year vs. a 1.9 line decrease. This was statistically significant (p=0.0014). It is important to realize this is a small group of patients and though this is an extremely encouraging result, whether this trend and statistical significance will be maintained when the study is completed is undetermined.
Secondary Endpoints
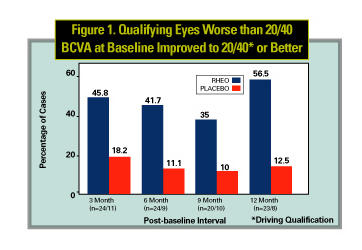
Other changes were also observed following rheopheresis therapy. At the one-year endpoint, for those patients who were less than 20/40 to begin with (legal limit for driving acuity), 57.9 percent of patients in the treatment group passed their driving qualification test compared with 14.3 percent of the non-treatment group. Mean improvement in reading test scores (Pepper Visual Skills for Reading Test, PVSRT) improved by 31 percent for the treatment group compared to 14 percent for the placebo group. Dramatic and statistically significant differences were also observed for the VFQ-25 score. The percent of eyes with drusen reduction diagnosed on fundus photos and fluorescein angiography was 35 percent for the treatment group compared with 14 percent for the non-treatment group.
Safety
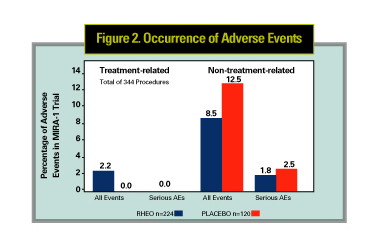
The safety of rheopheresis has also been assessed in large clinical trials in Europe as well as in the initial phases of the MIRA-1 study. In a large retrospective study of 1,791 treatments, the overall incidence of any adverse reaction was 4.9 percent, with paresthesias the highest at 1.5 percent and hypotension next at 0.65 percent. Figure 2 summarizes the results for the MIRA-1 study group for the treatment (224 procedures) and placebo (120 procedures) groups. Overall incidence of treatment-related adverse events were 2.2 percent for the treatment group and 0 percent for the placebo group. The most common adverse events for both groups were changes in blood pressure (hypotension in three and hypertension in four procedures in the treatment group, hypertension in three patients of the placebo group). No serious adverse events attributable to the treatment were reported.
Summary of Observations
The MIRA-1 study results to date suggest that:
1. Depletion of select high-molecular weight plasma components with rheopheresis induces a therapeutic effect that is evident immediately post-treatment, and the beneficial effect for best-corrected visual acuity for treated eyes is durable, remaining essentially stable throughout the 12- month study period.
2. The beneficial effects of rheopheresis are significant for all cohorts analyzed, and demonstrate significant improvement in BCVA (compared to placebo) at each study interval.
3. The proportion of eyes with improvement of >2 lines and >3 lines BCVA favors the treatment group. Consistent with this, the proportion of eyes with >2 and >3 lines of BCVA loss is lower for the treatment group. In addition, the effect is bilateral, owing to the systemic nature of the therapy.
4. Stratification of the data with subgroup analysis demonstrates that the timing of therapeutic intervention with rheopheresis in the course of AMD progression appears to be important, and may have a pronounced effect on BCVA outcomes.
5. Only those eyes with baseline BCVA of 20/40 to 20/125 had a significant beneficial effect from the treatment.
6. Eyes diagnosed with late-stage, high-risk pre-angiogenic AMD appear to be at significant risk for continued vision loss over the ensuing 12- month period if left untreated.
7. No eye in either cohort progressed to wet disease in 12 months. Of note, each study group was maintained on high levels of antioxidant supplements.
Compared to placebo, initial study results indicate that rheopheresis is a safe, effective and well-tolerated procedure that provides significant improvement in best corrected visual acuity for at least 12 months in select patients with high-risk dry AMD. Completion of the current clinical study (MIRA-1) should clarify any other long-term risks and benefits. Clinical and basic science studies will help clarify the cellular basis by which this therapy may provide some benefit.
Drs. Gallemore and Boyer are in private practice with the Retina-Vitreous Associates Medical Group in Beverly Hills, Los Angeles, North Hollywood and Torrance. Contact them at (310) 854-6201 or via e-mail at Retina2000@yahoo.com (RPG) and vitdoc@aol.com (DSB).



