The current treatment paradigms include tear substitutes, punctal plugs, autologous serum drops, topical steroids or calcineurin inhibitors.1 Despite some refinements in recent years, these therapeutic options provide limited relief for most dry-eye patients. At the same time, methods of clinical assessment have been slow to evolve with our understanding of dry eye as a disease of multiple etiologies and a long, diverse natural history. Further complications arise from complexities that can cloud efficacy measures, including large and variable placebo effects and compensatory mechanisms such as altered blink behavior.2
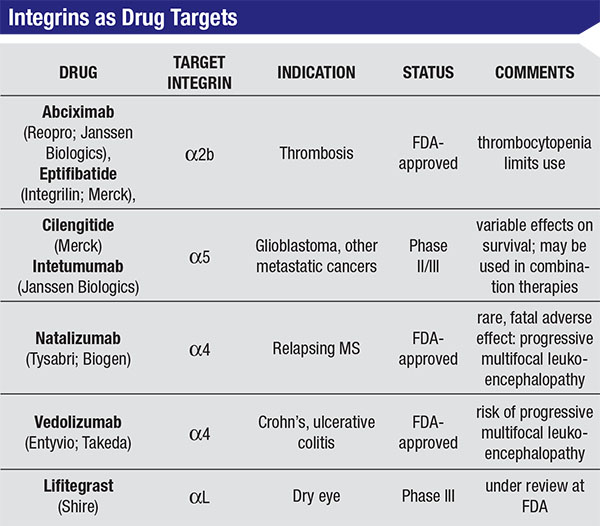
Efforts to surmount these two issues appear to have hit pay dirt with the recent clinical development of the integrin antagonist lifitegrast (Shire). Knowledge of immune-cell interactions led to the identification of a new drug target, while progress in clinical research helped to define patient populations in ways that allow for apples-to-apples clinical assessment and an optimal chance for a therapeutic breakthrough. This month, we will look at lifitegrast and examine its unique mechanism of action and the path that led to recent clinical success and an upcoming review by the Food and Drug Administration.3
Inflammation Is Key
In the search for therapeutic options it’s thought that one of the keys to a successful dry-eye treatment is to address the fundamental importance of inflammation in the disease,4,5 even though to date most anti-inflammatories have been found lacking in some way. Our best anti-inflammatory treatments, corticosteroids, act by diminishing expression of the cytokines and other signaling molecules, and thus dampening the entire response machinery. While they do provide relief for some patients, overall the response is mixed, and prolonged ocular steroid therapy is problematic.6 We are left to seek other targets in the inflammatory cascade for potential therapeutic intervention.
An inflammatory response can be initiated by any number of events, including trauma and bacterial or viral infiltration. (See the May 2014 Therapeutic Topics for an in-depth review of ocular inflammation.) The initial response awakens an intercellular protein complex called an inflammasome that converts preformed interleukin precursors, especially IL-1β and IL-6, to their active forms.7,8 These are released into circulation where they act as attractants for leukocytes, granulocytes and macrophages that congregate at the sites of insult. Immune cell recruits propagate and amplify the initial response with the release of additional cytokines, and also respond to chemo-attractants to move to the site of inflammation and initiate vasodilation and extravasation of immune cells out of circulation and into the inflamed tissues. This cycle is normally self-limiting, but in some conditions an inappropriate, chronic inflammatory state develops.
On the horizon, there are new therapies that target steps in this inflammation pathway, and one of the most promising of these is lifitegrast, a topically active compound that is specifically designed to disrupt the cell-to-cell interactions following the initial inflammatory trigger. The target for lifitegrast is Lymphocyte Function Antigen-1, first identified as a membrane protein required for cytotoxic T cell activity.9 The literature surrounding LFA-1’s importance is somewhat confusing because it was independently identified as LFA-1, as a dimeric surface marker of lymphocyte cell differentiation (CD11/CD18), and as the integrin dimer αLβ2.10,11 In understanding how lifitegrast can elicit an anti-inflammatory response, it’s most useful to consider the drug target as a member of the integrin family, a class of proteins that mediate cell-to-cell contact and communication.12,13
There are several protein families that have been implicated in the cell-to-cell communication that’s key to immune-cell recruitment: cell-adhesion molecules; selectins; and integrins.11-13 Through a combination of interactions between proteins on the immune cell surface and their surroundings, cells are mobilized to sites of inflammation response. This recruitment involves several specific, pairwise interactions between the cells and their surrounding endothelium. Movement of leukocytes and other cells through small arterioles and capillaries occurs by a rolling mechanism in which selectins bind and unbind with opposing glycoproteins at relatively low affinity; some integrins may also participate in this process. Key to this phase of the recruitment process is the low affinity that allows the cells to move over the endothelial surface.
When the rolling cell arrives at or near the site of trauma, local cytokine release has already been at work, increasing expression of endothelial cell adhesion molecules like ICAM-1 and V-CAM. These are the receptors for leukocyte integrins αLβ2 and α4β1. Surface expression of both ICAM-1 and V-CAM is upregulated by IL-1β and by interferon-γ,14,15 and the binding of ICAM-1 to αLβ2 is a high-affinity interaction that blocks the leukocyte rolling and triggers cytoskeletal rearrangements within the endothelial cell, leading to increases in permeability, breakdown of extracellular matrices and an increase in the access of leukocytes to interstitial space.16
|
Early work targeting this interaction focused on specific regions of each molecule involved in the binding interaction.19 Using these binding domains as a template, Thomas Gadek, PhD, currently CEO of Rogne Bioscience, and his colleagues tested a series of isoquinolones for their ability to interfere with LFA-1 binding to ICAM-1. One of these, designated SAR 1118, bound to LFA-1 and blocked subsequent interaction with ICAM-1 with IC50 concentrations in the 1 to 10 nM range.20 This action blocked infiltration of leukocytes, and attenuated release of inflammatory cytokines in vitro, including several that are elevated in dry eye in human tears: INF-γ; IL-1β; IL-6 and IL-10.21 SAR-1118 (now lifitegrast) is an excellent example of rational drug design: a compound whose structure was refined to optimize antagonist activity, and at the same time pharmacokinetic properties were tailored to best suit a topical medication. This yielded a drug with good ocular absorbance and rapid systemic elimination.20
From Lab to Clinic
Preclinical studies confirmed that lifitegrast was an effective anti-inflammatory, and specifically showed significant improvements in dogs diagnosed with KCS.18 Schirmer’s scores increased from a mean value (n=12) of 3.4 mm to 5.8 mm following 12 weeks of treatment. In Phase I studies, the safety of the drug was established, and in particular there was little systemic effect of the drug on lymphocyte markers or other measures of normal immune function.22 The only limitation to this study was that patients were primarily young (all were under 50, and 75 percent were under 40) and all were male. While this does not represent the typical dry-eye patient, results did confirm that the drug is safe up to doses of 5%.
Like all clinical studies of dry-eye treatments, Phase II and Phase III trials of lifitegrast faced the difficulty of a diverse pool of potential study patients and the high variability seen in both signs and symptoms of the disease. To address this, a Phase II dose-ranging study used ORA’s controlled adverse environment model.23 A total of 228 subjects participated in this prospective, multicenter, randomized, placebo-controlled, double-masked study, with patient inclusion enrichment based upon CAE responses. As part of the inclusion criteria, subjects for the study had to show a consistent exacerbation in both signs and symptoms of dry eye when exposed to the ocular stress of the CAE. The CAE consists of a specialized room (either a permanent site or a mobile unit) where humidity, temperature and air-flow are regulated. In this setting, potential subjects are exposed to environmental conditions associated with dry eye. Those who showed no evidence of worsening of signs and/or symptoms following exposure to the CAE were not eligible for enrollment in the study. Use of the CAE allows for a focus on those patients with the most reproducible disease. These patients represent an optimized study population that provides the best response window in which to assess treatment efficacy.
The primary endpoint was improvement in inferior corneal staining versus placebo at 12 weeks, and although the study did not meet this criterion for any of the three treatment groups, there was strong evidence of dose-dependent improvements in staining, and a consistent improvement in all groups compared with placebo. Inferior staining for both 1% and 5% lifitegrast was significantly improved when compared to baseline measures, and dose-dependent improvements in other secondary signs, such as Schirmer’s tests, were also significant.
Another promising result from the Phase II study was that symptomatic improvements in dry eye were also seen.22 Compared to baseline measures, dose-dependent improvements in ocular surface disease visual function scores were statistically significant (p<0.05) with both 1% and 5% lifitegrast.
In addition, the Ora ocular discomfort score also showed dose-dependent improvements and was significantly improved (p=0.044) for the group receiving the 5% dose.
With these encouraging results, the follow-up Phase III (OPUS-1) study looked at signs (inferior corneal staining) and symptoms (visual function component of OSDI) in a population of 588 dry-eye subjects.23 This trial used the same protocol used for the Phase II study, including CAE-based inclusion criteria and the Ora Calibra staining scales, a refined system of assessment optimized for measures of staining due to dry eye. Use of this grading scale involved training the investigators in the use of a metric distinct from traditional Oxford or National Eye Institute scales; in this way efficacy measures benefit from greater uniformity of assessment. The staining scores reflected this: mean change from baseline staining for 5% lifitegrast was highly significant at p=0.0208 (Phase II), and p=0.0007 (Phase III). While OSDI visual function improvements did not reach statistical significance, symptomatic improvements, including eye dryness (p=0.029) and Ora ODS (p=0.0273) were both significantly improved.
Collectively, the Phase II study and the OPUS-1 trial demonstrated a remarkable degree of reproducibility, a key goal for any drug development program and a particularly encouraging one for an indication such as dry eye, which is known for its variable nature. A second Phase III study with a larger patient population (n=720; NCT01743729) confirmed the ability of lifitegrast to provide clinically meaningful symptomatic relief for dry eye sufferers.
In recent years, a number of new therapies for dry eye have faced clinical hurdles because of the disconnect between signs and symptoms of the disease. This is what we’ve referred to as the enigma of dry eye. The development program exemplified by lifitegrast gives us hope that we may have cracked the code. Clearly, a better understanding of the underlying pathophysiology and a rational approach to development of new chemical entities can be critical elements to solving this puzzle. Equally important is a rigorous approach to clinical design, including CAE-based inclusion criteria and optimized clinical scales. These improvements should provide the therapeutic bandwidth necessary to decipher the challenge that is dry eye. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School, and emeritus surgeon at the Massachusetts Eye and Ear Infirmary. Mr. Ousler is vice president of dry eye at Ora Inc. Dr. McLaughlin is a medical writer at Ora Inc.
1. Alves M, Fonseca EC, Alves MF, Malki LT, Arruda GV, Reinach PS, Rocha EM. Dry eye disease treatment: A systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11:3:181-92.
2. Ousler GW, Abelson MB, Johnston PR, et al. Blink patterns and lid-contact times in dry-eye and normal subjects. Clin Ophthalmol 2014;8:869-74.
3. Shire Submits Application to the U.S. FDA for Approval of Lifitegrast for Treatment of Dry Eye Disease in Adults. [news release] Lexington, MA March 2, 2015. https://www.shire.com/newsroom/2015/march.
4. Hessen M, Akpek EK. Dry eye: An inflammatory ocular disease. J Ophthalmic Vis Res. 2014; 9:240-50.
5. Yagci A, Gurdal C. The role and treatment of inflammation in dry eye disease. Int Ophthalmol 2014;34:1291-301.
6. Coursey TG, de Paiva CS. Managing Sjögren’s syndrome and non-Sjögren’s syndrome dry eye with anti-inflammatory therapy. Clin Ophthalmol 2014;4:8:1447-58.
7. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013;13:397-411.
8. Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res 2015;134:133-40.
9. Sanchez-Madrid F, Krensky AM, Ware CF, et al. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA 1982;79:7489-93.
10. Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 1990;75:1037-50.
11. Springer TA, Dustin ML. Integrin Inside-Out Signalling and the Immunological Synapse. Curr Opin Cell Biol 2012;24:1:107–115.
12. Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci 2003;24:12:640-7.
13. Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunological Reviews 2007;218:135-146.
14. Yannariello-Brown J, Hallberg CK, Häberle H, et al. Cytokine modulation of human corneal epithelial cell ICAM-1 (CD54) expression. Exp Eye Res 1998;67:4:383-93.
15. Dustin ML, Rothlein R, Bhan AK, et al. Induction by IL-1 and interferon gamma: Tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1) distinct from LFA-1. J Immunol 1986;137:1270-1274.
16. Vicente-Manzanares M, Sánchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol 2004;4:2:110-22.
17. Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogren’s syndrome-like MRL/lpr mice. Experimental Eye Research 2004;78:823–835.
18. Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci 2011;52:3174−3180.
19. Gadek TR, Burdick DJ, McDowell RS, et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 2002;295:1086−1089.
20. Zhong M, Gadek TR, Minna Bui M. Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Med Chem Lett 2012;3:203.
21. Lam H, Bleiden L, De Paiva C, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 2009; 147:198.
22. Semba CP, Swearingen D, Smith VL, et al. Safety and pharmacokinetics of a novel lymphocyte function-associated antigen-1 antagonist ophthalmic solution (SAR 1118) in healthy adults. J Ocular Pharm and Ther 2011;27: 99-104.
23. Semba CP, Torkildsen GL, Lonsdale JD, et al. A phase II randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol 2012;153:1050-1060.
24. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: Results of the OPUS-1 phase III study. Ophthalmology 2014;121:475-483.