There are many lines of defense protecting the ocular surface from harm, beginning with reflex-gestures and brow line, and culminating with the eyelashes, lid, tear film and epithelium. Blinks, which last between 300 and 400 milliseconds, are integral to ocular surface health, as they help maintain the tear film.1 However, blinks are highly variable across tasks, such as reading, computer use, TV, driving, conversation and gazing.2 Blinking is also influenced by internal factors including fatigue, medications, stress and affective state.3 Clinically, it is crucial to consider the role of the blink when investigating ocular conditions, as behavior modifications can be important for treatment. This article will examine the task-dependent nature of the blink rate and its relationship to ocular surface conditions.
Physiology
The spontaneity of the eyeblink has piqued the curiosity of many scientists, and more than 80 years of research has been devoted to unraveling the mechanism behind the human eyeblink. Scientists have proposed various theories for the spontaneous nature of the blink: Some have suggested the presence of an internal blink pacemaker regulated by the brain stem,4 while others have alleged that the dopamine-related circuitry of the hypothalamus and of the caudate nucleus are involved.5,6 Research on non-human primates has demonstrated the direct relationship between cortical levels of dopamine and spontaneous blinking.7 The amplification of dopamine receptor 1 activity with D1 agonists has been shown to result in an increased blink rate. Correspondingly, blocking the dopamine receptor results in a decreased blink rate. Some researchers have also proposed that the blink rate is connected to mental load and is regulated by the rate of cognitive processing.8 According to a paper presented by State University of New York-Oswego researcher Kara Wallace at the XVth Biennial International Conference on Infant Studies in Kyoto, Japan, in 2006, speaking, memorizing and mental arithmetic have all been linked to an increased blink rate, while daydreaming, directing and redirecting one's attention and stimulus tracking have been associated with a decreased blink rate. The reason why certain cognitive processes and activities affect blink rate depends on the situation. Evolution-wise, the momentary closure of the eye can make the difference between a captured prey and a missed meal, which may explain the decreased blink rate during stimulus tracking.
A blink can be either a reflex response to corneal stimulation (e.g., irritation by a foreign body) or the result of stimulation of the supraorbital nerve. Blinks are carried out by a series of motor neuron activations. Activation of the orbicularis oculi (supplied by the zygomatic branches of the facial nerve, cranial nerve VII), the abducens (cranial nerve VI) and accessory abducens motor neurons, is responsible for closing the eyelids. The levator palpebrae motor neurons (innervated by the superior branch of the oculomotor nerve: cranial nerve III) lift the upper eyelid.9
The action of blinking not only protects the cornea and conjunctiva from external damage, it also enables the removal of debris from the ocular surface. Additionally, blinking allows for the distribution of tarsal goblet cell mucin and works to increase lipid layer thickness.10 Muscles of the lower lid margin—particularly the muscle of Riolan and the intratarsal muscle—are believed to be involved with controlling meibomian gland secretion.11 The lacrimal gland/oil layer reestablishes the tear film, but the tear film can only be maintained with adequate blink rate. Hence, adequate eyeblinks and an intact lid margin are critical to maintaining the ocular surface.
Exogenous Influences
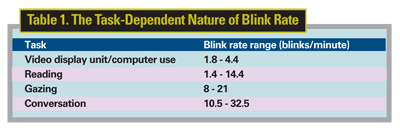
Blink rate can vary across tasks and states, and can increase in frequency and completeness. Indeed, even the intensity of illumination and glare has been shown to impact the frequency of blinking.12 Research into the task-dependent nature of the blink has supported blink-rate types in relation to four tasks: visual display unit use (e.g., computer); reading; primary gaze and conversation.1,13 These findings have been replicated across a series of experiments, demonstrating that one's blink rate is highly dependent upon experimental conditions. In a comprehensive literature review, Professor Michael Doughty, of Glasgow-Caledonian University in Scotland, highlighted the ranges of blinks per minute during reading as 1.4 to 14.4, primary gaze as eight to 21 and conversation as 10.5 to 32.5.2 Blink rates for visual display unit use have been suggested to fall within the range of 1.8 to 4.4 blinks per minute.13 The large ranges in blinks per minute are indicative of the high variability among subjects. Therefore, when measuring blink rate in a clinical setting, a researcher must tightly control environmental influences and task conditions to obtain relevant results.
Endogenous Influences
One's blink rate may also be influenced by internal factors, such as fatigue, stress, medications, ocular surgery and surface conditions. Research, including a paper presented at a meeting for the Society for Psychophysiological Research more than a decade ago, has demonstrated that fatigue and stress induce an increased blink rate in normals.14,15 Medications may also affect blink rate; for example, women on a birth control pill blink an average of 32 percent more than those not on the pill.16 Post-LASIK surgery patients may experience a reduced blink rate; this is partly the result of decreased corneal sensitivity.17 Ocular anesthetics can also cause decreased corneal sensitivity,3 which can lead to dry-eye disease and keratitis.
Dry Eye and Blink
Patients with dry eye can find their symptoms exacerbated by the additional ocular exposure caused by a decreased blink rate. A decreased blink rate causes an increase in the rate of tear-film breakup, along with a decrease in tear and mucin production and meibomian secretions in both normals and dry-eye patients with the disruption of tear-film components. As such, dry-eye patients are at a particular disadvantage during reduced-blink-rate activities, such as working on the computer, reading, driving, etc. Exacerbation of dry-eye signs and symptoms from a reduced blink rate can lead to visual function disturbances. We must keep in mind that a normal tear film protects the integrity of the ocular surface from keratitis and protects our vision: it is difficult to see through a broken tear film, especially with the presence of keratitis (Ousler GW et al. IOVS 2007;48: ARVO E-Abstract 410). Furthermore, a reduced tear-film breakup time (TFBUT) and the prolonged exposure of the ocular surface produce a disrupted air-fluid interface, which in turn disrupts light transmission to the retina. Hence, visual function is impacted.
Implications for Visual Function
Patients with ocular surface dysfunction are at a serious disadvantage when performing tasks that are typically linked to a prolonged interblink interval. This is not to say that patients with a short tear-film breakup time experience great discomfort during regular conversation; rather, ocular surface dryness and irritation are more likely exacerbated during tasks that require greater visual attention and therefore are associated with a lower blink rate. The dry-eye patient with a TFBUT of three seconds is at a specific disadvantage when working on a computer-oriented task, where the interblink interval can slow to as long as 12 seconds. Over the course of an hour, the ocular surface conditions may worsen, and keratitis may increase as a result of the successive, extended interblink intervals.
To date, it has been difficult to quantify the decay of visual acuity within the interblink interval. A new test, however, has been specifically developed and validated by our researchers at ORA Clinical Research and Development here in
Maintaining one's best-corrected acuity during the interblink interval is unlikely if the tear-film breakup is early and blink is late, with a significant time between blinks. Such an instance leads to an interval of decreased vision (Walker PM et al. IOVS 2007;48: ARVO E-Abstract 422). A study that investigated the relationship between central corneal staining and visual function in dry-eye patients used IVAD measurements to assess functional visual acuity in real time. Resulting data showed that patients with central corneal staining could not maintain their BCVA for as much time in between blinks as patients without central staining (Ousler III GW et al. IOVS 2007;48: ARVO E-Abstract 410). Consequently, problems can arise over time with rate of information recognition, perceptual processing, fatigue and function.
Future Research
Clinically, investigating blink rate in relation to patients with ocular surface conditions must be constrained within a specific task and meaningful to the functional outcome of the patient's everyday life (e.g., reading). If a relevant blink rate is not measured, as is frequently the case,2 no connection may be drawn between the patient's blink rate and the interference of ocular symptoms with everyday life.
Hence, researchers must make distinctions between blink rates. As no single "normal" blink rate exists, ranges of blink rates on specific tasks are a good starting point. It may be that a patient is an avid reader, a task associated with a long interblink interval. Awareness of the task-dependent nature of blink rates can greatly aid in treating patients with ocular surface conditions, who in turn require more frequent blinking to replenish the ocular surface. Ernest Lowenstein, OD, PhD, of the New England College of Optometry, has proposed a blinking exercise routine for such a purpose. Nevertheless, awareness of such blink patterns themselves can provide insight into the patient's daily routine and therefore the ability to suggest modifications, such as alternating long interblink interval tasks with shorter ones.
Further environmental influences must also be investigated. The association between scotopic vision and blink rates may also provide further insight into the underlying mechanisms. Blinking during tasks such as night driving may elucidate the role of external lighting influences on blink rate. The impact of air quality (i.e., the amount of irritants and pollutants that are present) must also be considered in relation to the dynamic blink rate.
The ability of drug therapies to treat individuals with ocular surface conditions and normalize their blink rate can have an enormous impact on their quality of life. Therapies that can extend tear-film breakup time can provide a protected ocular surface during the interblink interval, allowing dry-eye patients to go about their daily lives without having to worry about ocular discomfort, irritation or blurring.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Carney LG, Hill RM. The nature of normal blinking patterns. Acta Ophthalmol 1982;60:3:427-433.
2. Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: During reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci 2001;78:10:712-725.
3. Naase T, Doughty MJ, Button NF. An assessment of the pattern of spontaneous eyeblink activity under the influence of topical ocular anaesthesia. Graefe's Arch Clin Exp Ophthalmol 2005;243:306-312.
4. Davson H. The protective mechanisms. In: Davson H, ed. The Physiology of the Eye, 3rd ed.
5. Karson CN, Berman KF, Kleinman J, Karoum F. Seasonal variation in human central dopamine activity. Psychiatry Res 1984;11:2:111-117.
6. "Eye, human." Encyclopædia Britannica. 2007. Encyclopædia Britannica Online. 17 Apr. 2007 <http://www.britannica.com/eb/article-64887>.
7. Taylor JR, Elsworth JD,
8. Hollan M, Tarlow G. Blinking and mental load. Psychol Rep 1972;31:1:119-127.
9. Van Ham JJ & Yeo CH. Trigeminal inputs to eyeblink motorneurons in the rabbit. Experimental Neurology. 1996;142:2:244-257.
10. McMonnies CW. Incomplete blinking: Exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens Anterior Eye 2007;30:1:37-51.
11. Iwanami M, Tsurukiri K. Histological comparison between young and aged specimens of the Oriental lower eyelid using sagittal serial sections. Plast Reconstr Surg 2007;119:7:2061-71.
12. Luckiesh M, Moss FK. The eyelid reflex as a criterion of ocular fatigue. J Exper Psychol 1937;20:589-596.
13. Patel S, Henderson R, Bradley L, Galloway B, & Hunter L. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci 1991;68:111:888-892.
14. Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, & Rinaldi F. Diurnal variation in spontaneous eye-blink rate. Psychiatry Research 2000;93:2:145-151.
15. Tecce J. (1989). Eyeblinks and psychological functions: A two-process model. Abstract: Psychophysiology;26:4A(S):5-6.
16. Yolton DP, Yolton RL, Lopez R, Bogner B, Stevens R, & Rao D. The effects of gender and birth control pill use on spontaneous blink rates. J Am Optom Assoc 1994;65:11:763-770.
17. Moshirfar M, Marx DP, Barsam CA, Mohebali J, & Mamalis N. Salzmann's-like nodular degeneration following laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:10:2021-2025.



