Treating glaucoma is currently an imperfect science, for a simple reason: Our understanding of aqueous production and outflow is incomplete. To understand why a biological system breaks down, you must first understand how the system works when it's healthy. The more accurate our model of the system, the more effective our treatments and the less vision lost.
There's good reason to suspect that the currently accepted model of aqueous outflow is inaccurate. The traditional model portrays the trabecular meshwork as a passive filter: a rigid matrix of extracellular material through which aqueous passes, regulating pressure and flow via an unchanging resistance. Five pieces of evidence argue against this model:
• The meshwork is not a static or rigid matrix. There is abundant evidence that the entire meshwork moves; it is deformed as intraocular pressure increases and then rebounds as pressure decreases. This was first systematically demonstrated by Martin Grant and myself1 and explored in remarkable detail by Ian Grierson and William Lee2-4 shortly thereafter. A passive filter requires a rigid syncytium of tissue with unchanging geometry to effectively permit it to function as a resistance unit.5
• Aqueous movement into the episcleral veins from Schlemm's canal is pulsatile. A rigid passive filter would not be capable of converting the normal intraocular pulse into a wave of aqueous originating from Schlemm's canal. However, clinical in vivo studies document pulsatile flow from the anterior chamber into Schlemm's canal,6,7 from the canal into collector channels,6,7 and from the collector channels into aqueous and episcleral veins6-12—all synchronous with the ocular pulse. (The highest quality evidence is obtained from such direct observation because no assumptions about the nature of tissue and fluid movement are required.) 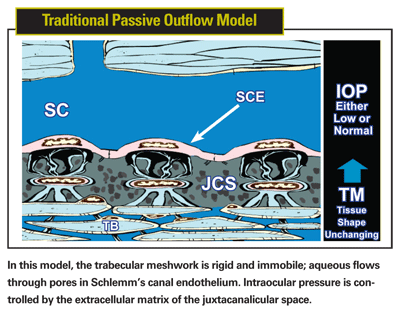
The observation that aqueous outflow pulsates is not new. Karl W. Ascher13,14 and Hans Goldmann,12 who independently discovered the aqueous veins, noted such pulsations in their original descriptions and documented the pulsatile flow in great detail. However, they were unsure what to make of the pulsatile flow because they accepted the traditional model of the meshwork as a passive filter. Ascher, in his comprehensive 1961 book The Aqueous Veins8 noted that he couldn't explain how an intraocular pulse generated inside the eye could cross a rigid Schlemm's canal endothelium and generate a pulse, driving fluid into the aqueous veins. As a result, this significant observation was largely ignored for 50 years because there was no framework to account for it.
• Active valves are visible. Diverse lines of laboratory, operating room and clinical evidence indicate the presence of tiny cellular valves that permit aqueous flow into Schlemm's canal.6,7,15 Gonioscopic examination provides the highest quality of evidence: direct observation. Pulsatile aqueous flow through these valves into Schlemm's canal may be seen in the eye of a living human, as documented by Stegmann.6,7 (To see a video of this—courtesy of Robert Stegmann—visit youtube.com/majohnstone on the Web.)
• A passive filter can't regulate pressure and flow. The trabecular meshwork acts as a regulatory framework, ensuring that pressure and flow are regulated and homeostasis is maintained. Glaucoma occurs because of derangement of this regulatory system.5,16,17 A rigid, passive extracellular filtering system in the juxtacanalicular space provides no mechanism for transferring intraocular pressure gradients to the appropriate living tissues so they can sense and respond to changes in pressure and flow.
• The TM doesn't provide the amount of resistance described. Maintaining pressure inside the eye requires a level of resistance that is not provided by the extracellular matrix and juxtacanalicular space acting as a filter, because at physiologic pressure the space is large and contains sparse amounts of extracellular matrix. Several studies have shown this.1,2,4,18,19
Other, more general arguments against the old model can be made as well. For example, in nature nothing is wasted. But according to the old model, most of the structural elements of the trabecular meshwork serve no purpose. The only region doing anything important is the juxtacanalicular space, which is represented as acting as a filter. Schlemm's canal endothelium is described as a low resistance system unable to act to restrict flow. The trabecular beams (lamellae), which are the predominant feature of the trabecular meshwork, and the cells surrounding them, are relegated to nonfunctional status.
Instead: A Biomechanical Pump
The aqueous system is part of the body's vascular system. The ciliary processes produce aqueous as a derivative of the bloodstream, and the aqueous eventually returns to the bloodstream via the TM, Schlemm's canal, and aqueous and episcleral veins. So it seems reasonable that the aqueous outflow system might be analogous to other vascular loops like the venous system and lymphatics. These vascular loops return fluid to the heart using a series of one-way valves that respond to pressure transients. An analagous aqueous pump model makes perfect sense in light of the evidence listed above.
In this biomechanical pump model,6,7 aqueous is actively moved into Schlemm's canal by a one-way pump composed of cellular and extracellular elements. These elements provide a regulatory feedback system that controls aqueous flow and intraocular pressure. The pump is powered by transient increases in IOP, primarily those caused by the cardiac cycle, blinking and eye movements.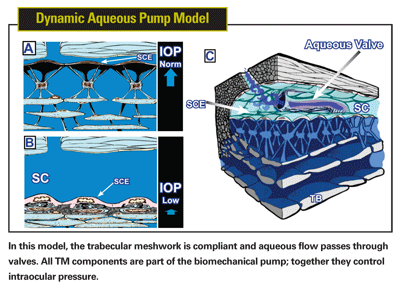
The pressure changes produce microscopic deformation in the inner wall endothelium of Schlemm's canal and the trabecular meshwork. As intraocular pressure increases, fluid is forced into one-way collector valves that span across Schlemm's canal. At the same time, the intraocular pressure increase pushes the inner endothelial wall of Schlemm's canal outward, closer to the external canal wall. The outward movement compresses Schlemm's canal lumen and forces aqueous in the canal to move circumferentially into collector channels and aqueous veins. As the pressure spike decays and pressure drops, the tissues rebound, causing a pressure drop inside Schlemm's canal, moving fluid from the one-way valves into the canal to compensate. (See figure, below.)
In terms of maintaining homeostasis, the aqueous pump provides short-term pressure control by varying stroke volume in response to pressure changes; it provides long-term pressure control by modulating TM constituents that control stroke volume. Trabecular endothelial cells sense both pressure and flow, providing a feedback mechanism allowing them to modulate the TM constituents. Comparable regulatory biomechanical linkages (mechanotransduction mechanisms) are present throughout the vascular system.
As noted, this model is supported by a growing body of evidence—some of which has been observed (but unexplained) for decades. And unlike the passive model, in the biomechanical pump model, all the structural elements of the trabecular meshwork have functional significance, including the trabecular lamellae, juxtacanalicular cells, the inner wall endothelium and the little valves crossing the canal. Structure and function are tied together.
Actually, aspects of this new model have been suggested for over 30 years. However, as typically happens in science, an accepted paradigm takes time to change, regardless of the evidence. (For a more detailed description of the biomechanical processes involved in this model and extensive supporting literature, see references #6 and #7.)
Modeling Glaucoma
In the passive filter model of aqueous outflow, glaucoma is thought to be caused by an excess accumulation of extracellular matrix material—i.e., an abnormality in the juxtacanalicular space. In the pump model of outflow, the explanation for system breakdown is completely different; something interferes with the efficiency of the pumping mechanism.
For example, the biomechanical pump model depends on tissue compliance rather than resistance. So, if the tissue loses its ability to move, the system begins to fail. Failure also occurs if Schlemm's canal walls remain in apposition for too long. Clearly, if the internal and external walls of the canal are pushed together and not allowed to rebound, fluid movement will be blocked. Furthermore, if the tissues remain in apposition for an extended period of time without movement, they're likely to stiffen and the walls may eventually become adherent. (An analogy would be angle-closure glaucoma, in which the iris is held in place against the trabecular meshwork and eventually becomes adherent.) Robert Stegmann, MD, who has had extensive experience unroofing Schlemm's canal,20 emphasizes that in glaucoma the meshwork is stiff, and in advanced glaucoma the walls of Schlemm's canal can be very difficult to separate.
Treating glaucoma, according to this model, could be approached by several means: enlarging the canal lumen; reversing structural abnormalities (for example, reducing excess rigidity of TM tissues to allow them to rebound after a pressure spike); or simply increasing the effectiveness of aqueous pumping. In fact, several studies now suggest that many pharmaceuticals may be doing the latter.
The Pharmaceutical Connection
If the new model is correct, it may serve to provide better explanations for the effectiveness of familiar glaucoma drugs, as well as providing new targets for therapeutic intervention. Both Goldmann and Ascher in their initial studies noted a blood and aqueous stratum where aqueous enters the episcleral veins. The diameter of the pulsating aqueous strata of the aqueous veins oscillates in synchrony with the ocular pulse. Multiple studies have documented the effect of different pressure-reducing drugs on the velocity and amplitude of the oscillatory aqueous pulse wave.8 For example, early studies documented such behavior with both epinephrine9,10,12 and pilocarpine.10,11,8
A study conducted by Elizabeth Martin, Annisa Jamil, Selene Isaacson and myself found that brimonidine causes pulse amplitude and velocity to increase greatly. We used a microvascular microscope to measure the amplitude of the flow through aqueous veins in eight eyes of eight normal subjects. (Where aqueous enters the episcleral veins, separate strata of aqueous and blood form.)
We found that before brimonidine the mean aqueous strata diameter was 24 ±16 µm; at 60 minutes after instillation the mean ASD was 45.9 ±22.4 µm—a 91-percent increase. (Increased aqueous flow into the aqueous vein was usually visible within five minutes of instillation.) We also measured the change in velocity in three subjects. We found that at maximum velocity the increase was 87 percent, 6 percent and 88 percent, respectively. (These results parallel the pulse amplitude and velocity effects previously documented with epinephrine and pilocarpine.)
We presented another paper at the 2007 Association for Research in Vision and Ophthalmology meeting, profiling a study showing that latanoprost also increases pulsatile flow [MA Johnstone et al; IOVS 2007 48: ARVO E-Abstract 1553]. We made ten measurements of the maximum aqueous strata diameter after latanoprost instillation in five subjects and measured aqueous flow velocity in two of them.
Increased amplitude and velocity of the oscillating pulse wave of the aqueous strata first appeared between 10 and 30 minutes after instillation, and was marked at 60 minutes. Before latanoprost, mean maximum strata diameter during systole was 30.3 ±6.5 µm; 60 minutes after instillation, the mean was 60.5 ±19.2 µm—a 100-percent increase. In the two subjects in whom we measured flow velocity, the flow increased by 68 percent and 37 percent respectively at 60 minutes.
Latanoprost's effect on the aqueous pump mechanism may be different from the other agents mentioned. Unlike the effect of brimonidine, episcleral vein diameter did not change following latanoprost instillation. Rather, four studies21-24 document that latanoprost increases the ocular pulse amplitude by about 20 percent, an effect absent with brimonidine.23 Such an increase in ocular pulse amplitude is likely to increase the effectiveness of the aqueous pumping mechanism pretty dramatically. 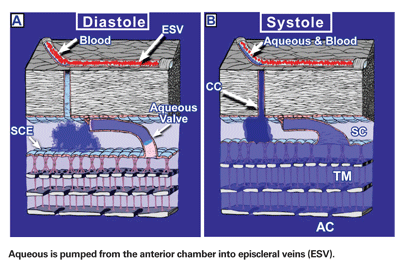
One of the studies suggested that "the increase in pulsatile ocular blood flow might be caused by vasodilatation in the choroid."21 Vasodilatation in the choroid would cause the incoming cardiac pulse wave to have a greater effect, thus increasing the impact of the choroidal piston that drives the ocular pulse.
In summary, we now have documentation that three different classes of medication increase the effectiveness of the aqueous pumping mechanism. If this is, in fact, how these agents work, this new perspective may not only help us to maximize their effectiveness but may open up a host of new avenues for research and development.
Better Model, Better Treatments
Finding a true cure for glaucoma depends, among other things, on having an accurate model of how aqueous outflow functions. At this point, a substantial body of evidence suggests that the passive filter model is not accurate. On the other hand, the biomechanical pump model accounts nicely for a large body of existing laboratory and clinical data. At the same time, it provides an explanation for a number of things that have previously been unexplained, it raises new questions, and it provides a new series of targets for treatment.
That can only be a good thing.
Dr. Johnstone has held positions as assistant professor of ophthalmology and consultant in glaucoma at the
1. Johnstone MA, Grant WM. Pressure-dependent changes in structure of the aqueous outflow system in human and monkey eyes. Am J Ophthalmol 1973;75:365-83.
2. Grierson I, Lee WR. Changes in the monkey outflow apparatus at graded levels of intraocular pressure: A qualitative analysis by light microscopy and scanning electron microscopy. Exp Eye Res 1974;19:1:21-33.
3. Grierson I, Lee WR. Pressure-induced changes in the ultrastructure of the endothelium lining of Schlemm"s canal. Am J Ophthalmol 1975;80:5:863-84.
4. Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8-30 mmHg). Exp Eye Res 1975;20:6:505-21.
5. Ellingsen BA, Grant WM. The relationship of pressure and aqueous outflow in enucleated human eyes. Invest Ophthalmol 1971;10:6:430-7.
6. Johnstone MA. The aqueous outflow system as a mechanical pump: Evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma 2004;13:5:421-38.
7. Johnstone MA. A New Model Describes an Aqueous Outflow Pump and Causes of Pump Failure in Glaucoma. In: Grehn F, Weinreb RN, eds. Essentials in Ophthalmology: Glaucoma, First ed.
8. Ascher KW. The Aqueous Veins. Vol. 1.
9. Ascher KW. Glaucoma and the aqueous veins. Am J Ophthalmol 1942;25:11:1309-15.
10. Ascher KW. Local pharmacologic effects on aqueous veins. Am J Ophthalmol 1942;25:1301.
11. De Vries S. De Zichtbare Afvoer Van Het Kamerwater, 1st ed.
12. Goldmann H. Abfluss des Kammerwassers beim Menschen. Ophthalmologica 1946;111(Feb.-Mar.):146152.
13. Ascher KW. Aqueous veins. Am J Ophthalmol 1942;25:31.
14. Ascher KW. Physiologic importance of the visible elimination of intraocular fluid. Am J Ophthalmol 1942;25:1174-209.
15. Johnstone MA. Pressure-dependent changes in configuration of the endothelial tubules of Schlemm"s canal. Am J Ophthalmol 1974;78:4:630-8.
16. Grant WM. Facility of flow through the trabecular meshwork. Archives of Ophthal. 1955;54:245-8.
17. Grant WM. Experimental Aqueous Perfusion in Enucleated Human Eyes. Archives of Ophthal. 1963;69:783-801.
18. Gong H, Ruberti J, Overby D, et al. A new view of the human trabecular meshwork using quick-freeze, deep-etch electron microscopy. Exp Eye Res 2002;75:3:347-58.
19. Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest Ophthalmol Vis Sci 1982;22:5:625-32.
20. Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999;25:3:316-22.
21. Georgopoulos GT, Diestelhorst M, Fisher R, et al. The short-term effect of latanoprost on intraocular pressure and pulsatile ocular blood flow. Acta Ophthalmol Scand 2002;80:1:54-8.
22. Geyer O, Man O, Weintraub M, et al. Acute effect of latanoprost on pulsatile ocular blood flow in normal eyes. Am J Ophthalmol 2001;131:2:198-202.
23. Liu CJ, Ko YC, Cheng CY, et al. Effect of latanoprost 0.005% and brimonidine tartrate 0.2% on pulsatile ocular blood flow in normal tension glaucoma. Br J Ophthalmol 2002;86:11:1236-9.
24. McKibbin M, Menage MJ. The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucoma. Eye 1999;13 (Pt 1):31-4.