In 2022, three advanced-technology IOLs were approved: Bausch + Lomb’s IC-8 Apthera, Lenstec’s ClearView 3 and Johnson & Johnson Vision’s Tecnis Symfony OptiBlue. Each lens was designed in a unique way to provide physicians with more IOL options to recommend to their patients. We spoke with surgeons who work closely with each lens to discuss their experiences working with the IOLs and what they’ve observed since the FDA approvals.
IC-8 Apthera Small Aperture IOL
 |
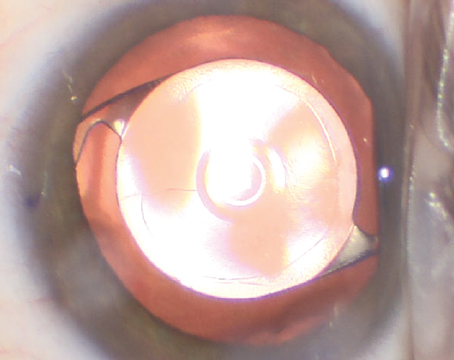
| The Apthera IOL is equipped with the carbon black FilterRing in the center. (Courtesy Apthera) |
Bausch + Lomb acquired AcuFocus to continue advancements on extended-depth-of-focus IOLs. The IC-8 Apthera IOL is a non-toric IOL indicated for patients with up to 1.5 D of corneal astigmatism. What makes this lens unique is its small aperture design using the AcuFocus FilterRing. Basically, the aperture was designed to filter out defocused or aberrated light that degrades image quality. The lens is available in powers of +10 D to +30 D in 0.5-D increments. Additionally, the IOL is packaged with a 3.5 mm injector system.
“In this iteration, Apthera provides about 0.91 D of depth of focus or defocus over a monofocal,” says Elizabeth Yeu, MD, of Virginia Eye Consultants in Norfolk. She says, “If you actually look at it it’s also approved to be used for monovision.” The Apthera was designed to be implanted in the non-dominant eye, supported by a monofocal or monofocal toric IOL implanted in the dominant eye.
During clinical trials, 343 subjects had an Apthera implanted in one eye and a monofocal IOL implanted in the other eye. In the control group, 110 subjects had monofocal IOLs implanted in both eyes. Two subjects in the Apthera group had their IC-8 IOL removed after a 12-month period due to adverse reactions. Between the two groups, 83.6 percent of Apthera subjects achieved a UCNVA of 0.30 logMAR (20/40) or better compared with 33 percent of subjects in the control group. In terms of distance vision, 89.6 percent of Apthera subjects achieved a UCDVA of 0.10 logMAR (20/25) or better compared with 92 percent of subjects in the control group.
Dr. Yeu implanted 27 Apthera IOLs during the trials. Since the trial, she reports that none of these patients need to use reading glasses anymore. However, a monovision strategy using the IC-8 isn’t suitable for all patients. Dr. Yeu says, “I do have one patient who says when they look out of the eye with the Apthera lens alone they do notice some dimness in nighttime driving, or it feels a little weird with their depth perception out of that eye.”
The IC-8 Apthera IOL is meant to be implanted in the capsular bag of a single eye, but Dr. Yeu indicates that the treatment could be done bilaterally. “There are definitely surgeons who have implanted the Apthera lens in both eyes off label, but this is a major conversation to have with your patients,” she says. “It’s the next level of consideration, because that’s going to have different types of implications for what would happen in terms of potential contrast sensitivity concerns.”
Dr. Yeu notes that the Apthera is a “forgiving” lens for patients with abnormalities or irregularities in their corneas. Dr. Yeu estimates that about 75 percent of her Apthera patients have cornea irregularities. “It’s an off-label indication, but I still do it in a monovision fashion where I’m offsetting the Apthera to give patients more near range,” she says. “For example, a recent patient of mine had hand motion vision for the last 20 years as his RK created such an irregular astigmatism. I could see that by using a small aperture of a sub-2 mm pupil, the light would create an image quality that would be 20/30. I looked at the results and found that he has 20/20 distance vision, all the way through to J1 vision.”
Dr. Yeu has seen positive results from her patients. She says that “patient satisfaction is even more overwhelming because of where they start. They start from not being able to see anything with any level of satisfaction or quality in spectacle correction or soft contact lens correction, to having uncorrected vision in the eye with the Apthera lens.”
ClearView 3 Multifocal IOL
 |
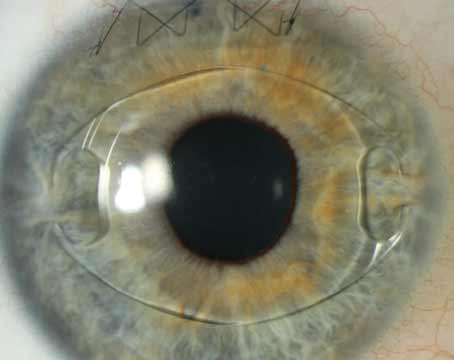
| ClearView 3’s bifocal design features a superior optic for distance vision and an inferior optic for near vision. (Courtesy LensTec) |
The ClearView 3 Segmented Bifocal Lens from Lenstec was originally called the SBL-3. This is an asymmetric, segmented, multifocal IOL offered in the dioptric power range of +15 D to +25 D in 0.25-D increments, and +25.5 D to +30 D in 0.5-D increments.
During clinical trials, 476 subjects had an IOL implanted in at least one operative eye: 315 subjects had the ClearView 3 implanted and 161 subjects in the control group received a monofocal IOL. The company says that persistent adverse reactions in the ClearView 3 group weren’t significant. Only one case of corneal stromal edema and one case of cystoid macular edema were observed. Investigators reported that the mean DCNVA in the ClearView 3 group was 0.109 logMAR (~20/25) while the control group was 0.569 logMAR (~20/80). Additionally, the mean BCDVA in the ClearView 3 group was 0.003 logMAR (~20/20) while the control group was -0.039 logMAR (~20/20), which didn’t represent a statistically significant difference.
T. Hunter Newsom, MD, founder and medical director of Newsom Eye in Florida, was an investigator for the ClearView 3 trials. Since then, he has been informing surgeons about the latest advancements in multifocal technology with the ClearView 3. “The ClearView lens isn’t a diffractive IOL technology. It’s not splitting the light like we’re thinking with the current technology. It’s like a progressive pair of glasses, with a monofocal distance lens on the top half with a transition zone, and then a monofocal near lens on the bottom half. It’s more like one monofocal on top of another monofocal.”
Although the segmented bifocal design of ClearView 3 resembles the design of bifocal spectacles, clinical trials indicate that patients implanted with the IOL don’t need to move their heads up and down to gain the advantage of near vision. Patients’ brains can adapt to suppress images out of focus from the lens, similar to other approved dual-powered multifocal IOLs. The ClearView 3 is meant to promote less frequent use of vision correction choices at near distance, therefore an efficient transition between distance and near is integral to the design.
To implant the ClearView 3, Lenstec provides different injectors and cartridges for various diopter ranges. The IOL is compatible with Lenstec’s I-9011S and I-9012 injectors, and it comes with a disposable cartridge from the LC 16 series. “The injectors are really easy to use. The haptics and lens are simple and easy to fold and inject,” says Dr. Newsom.
The ClearView 3 is reported to have very limited amounts of visual side effects. According to Dr. Newsom, the side effects of the ClearView 3 are similar to those of progressive lenses. “There can be some distortion in ClearView 3 lenses,” he says, “but if patients have already experienced that distortion with a pair of progressive glasses, then they’ve already tried this technology before they invest in the IOL.”
Tecnis Symfony OptiBlue IOL
 |
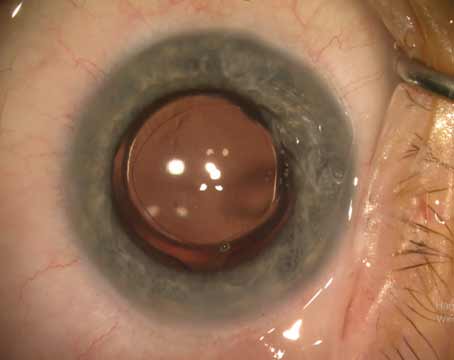
| The latest Tecnis Symfony IOLs from the InteliLight portfolio, OptiBlue and Synergy, are both available with the disposable Tecnis Symfony injector system. (Courtesy J&J Vision) |
Since the introduction of the Tecnis Symfony IOL in 2016, Johnson & Johnson Vision has been working towards advancing the technology of the IOL, while reducing halos, glare and starbursts. Also included in the Tecnis portfolio is a toric option.
During clinical trials, 148 subjects had the Symfony implanted bilaterally and another 151 subjects had a monofocal IOL implanted bilaterally. Overall, 2.7 percent of Symfony subjects experienced serious adverse reactions during the trial. None of the subjects experienced any device-related complications or unanticipated events. After six months, investigators found no persistent adverse reactions from the Symfony. However, one Symfony subject was diagnosed with CME after six months, but the investigators ruled this insignificant to the overall trial. Additionally, 76.9 percent and 70.1 percent of Symfony eyes achieved UCIVA and DCIVA of 20/25 or better, respectively, compared to 33.8 percent and 13.5 percent of monofocal eyes.
Douglas Grayson, MD, medical director of Omni Eye Services in Iselin, N,J., has been working with the Tecnis portfolio since its initial release. “The major advantages of Symfony when it came out was that it wasn’t considered a multifocal lens, it was an extended-depth-of-focus lens. Although, it did have the design of a multifocal,” he says. “The original Symfony didn’t have the OptiBlue coating, so there were patients who reported strange visuals at nighttime when they were driving and staring at headlights.”
In 2022, J&J introduced the Tecnis Symfony OptiBlue IOL with InteliLight technology, also available in a toric option. This new technology was first introduced in the Tecnis Synergy IOL, a hybrid lens. InteliLight combines a violet-light filter, an echelette design and achromatic technology to enhance the overall visual experience for patients, the company says. The violet-light filter was added to block short wavelengths of light that produce light scatter in the eye. This helps to mitigate halos, glare and starbursts, especially while driving at night, the company says. J&J adds that the echelette design helps mitigate halos and starbursts when interacting with a digital device, and the achromatic technology corrects chromatic aberration for better contrast day and night.
“OptiBlue can be loaded in a separate cartridge which can then be inserted through a 2.4-mm wound. The Symfony OptiBlue has the advantage of also being available in a preloaded injector using a well-designed disposable cartridge and injector system,” says Dr. Grayson. J&J’s disposable injector, Tecnis Simplicity, can be ordered along with the OptiBlue and toric option to prevent manual loading errors.
The OptiBlue IOL does pose some side effects, but the latest advancements didn’t enhance any adverse reactions, according to Dr. Grayson. “I don’t think there was any detriment to the product. I don’t think it created any other side effects,” says Dr. Grayson. What he did notice about the design of the OptiBlue IOL was that it affected the quality of night vision. “You don’t have the same type of night vision that you would gain from a monofocal lens,” he says. “The Symfony is all based on concentric rings, and if you look at a point source of light, you can get some of the glare effect or halo effect.”
Regarding reading vision, Dr. Grayson says, “There’s also the whole issue of how well patients are reading. If they’re paying additional money for a presbyopia correcting lens, then they’re expecting to read. Symfony OptiBlue doesn’t give the type of small J1 reading that some other IOLs can provide, but the upside is that the OptiBlues are a well tolerated lens.
“If a patient has the desire to be as spectacle independent as possible in both eyes for distance and near, then they’re a potential candidate for this multifocal. As long as there’s no significant eye pathology precluding the success of the lens, then the patient will generally do well with the OptiBlue,” adds Dr. Grayson. According to clinical trials, OptiBlue is meant for cataract patients with the potential for postoperative BCDVA of 20/30 Snellen or better.
After working with various IOLs, Dr. Grayson notes that the reason patients may prefer other vision correcting options doesn’t have to do with the IOL side effects. “The single biggest reason patients don’t like multifocal lenses is that there’s an issue with the power calculation or the toric axis calculation of the lens that was put in. It’s not so much the lens, it’s the calculation for the lens,” he says. “The gold standard is to be able to see distance, intermediate and near. It seems like the best way to achieve that is to take the design of these IOLs and try to continue modifications to try to minimize whatever symptoms remain.”
Dr. Yeu is a consultant for Bausch + Lomb and Johnson & Johnson Vision. Dr. Newsom is a consultant and principal investigator for Lenstec. Dr. Grayson has no financial interest in the products discussed.